Supported by the industry, such immunomodulatory supplements have found their way into routine perioperative care in abdominal surgery. Yet, their effect on clinically relevant outcomes like mortality, morbidity, and hospital stay remains unclear, mainly due to vast heterogeneity across study results and considerable overall and industry bias. Level-one evidence including two large-scale meta-analyses of randomized clinical trials (RCTs) challenge the beneficial role of immunonutrition in open and particularly also minimally invasive major abdominal surgery (2, 3).
The potential benefit of Immunonutrition
Patients undergoing major abdominal surgery are subject to significant surgical trauma and frequently present with impaired nutritional status (4). This aggravates the surgical stress response and compromises immune function and tissue repair, putting them at considerable risk for postoperative morbidity (5-7). In this context, nutritional interventions including immunonutrition therefore appear to be particularly promising. Formulae enriched with immunomodulatory substrates such as amino acids (arginine and/or glutamine), omega-3 fatty acids, and nucleotides or ribonucleic acid (RNA) have been shown to attenuate the inflammatory response (1). Collectively, these substances increase the nitrogen balance and protein synthesis, improve proliferation and function of lymphocytes and macrophages, alleviate the cytokine storm, maintain the intestinal barrier integrity, and enhance antioxidant defense mechanisms (8-10). In the era of enhancing recovery after surgery, supplementing perioperative nutrition with these substances has been advocated by many and has become an integral part of various perioperative protocols.
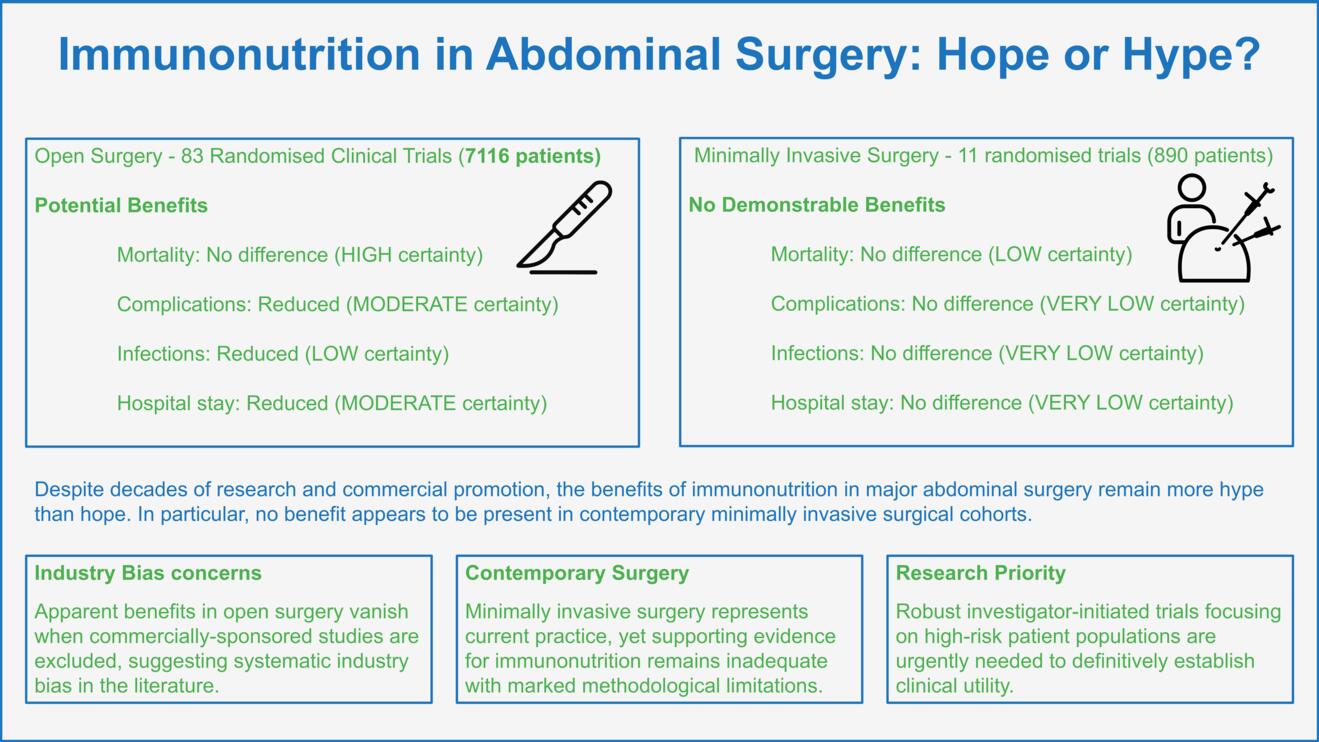
Current international guidelines on clinical nutrition in surgery recommend the perioperative (5-7 days pre- and/or postoperatively) use of immunonutrition in patients at nutritional risk, specifically malnourished general surgery patients and those undergoing major cancer surgery (5). This statement is primarily based on randomized data demonstrating reduced overall complications and shortened hospital stay for patients treated with immunomodulatory supplements. However, despite these possible benefits, considerable limitations in the current body of evidence challenge these conclusions. Two studies comprehensively synthesized the body of evidence in both open and minimally invasive major abdominal surgery (Table 1) (2, 3).
Current Evidence in Open and Minimally Invasive Major Abdominal Surgery
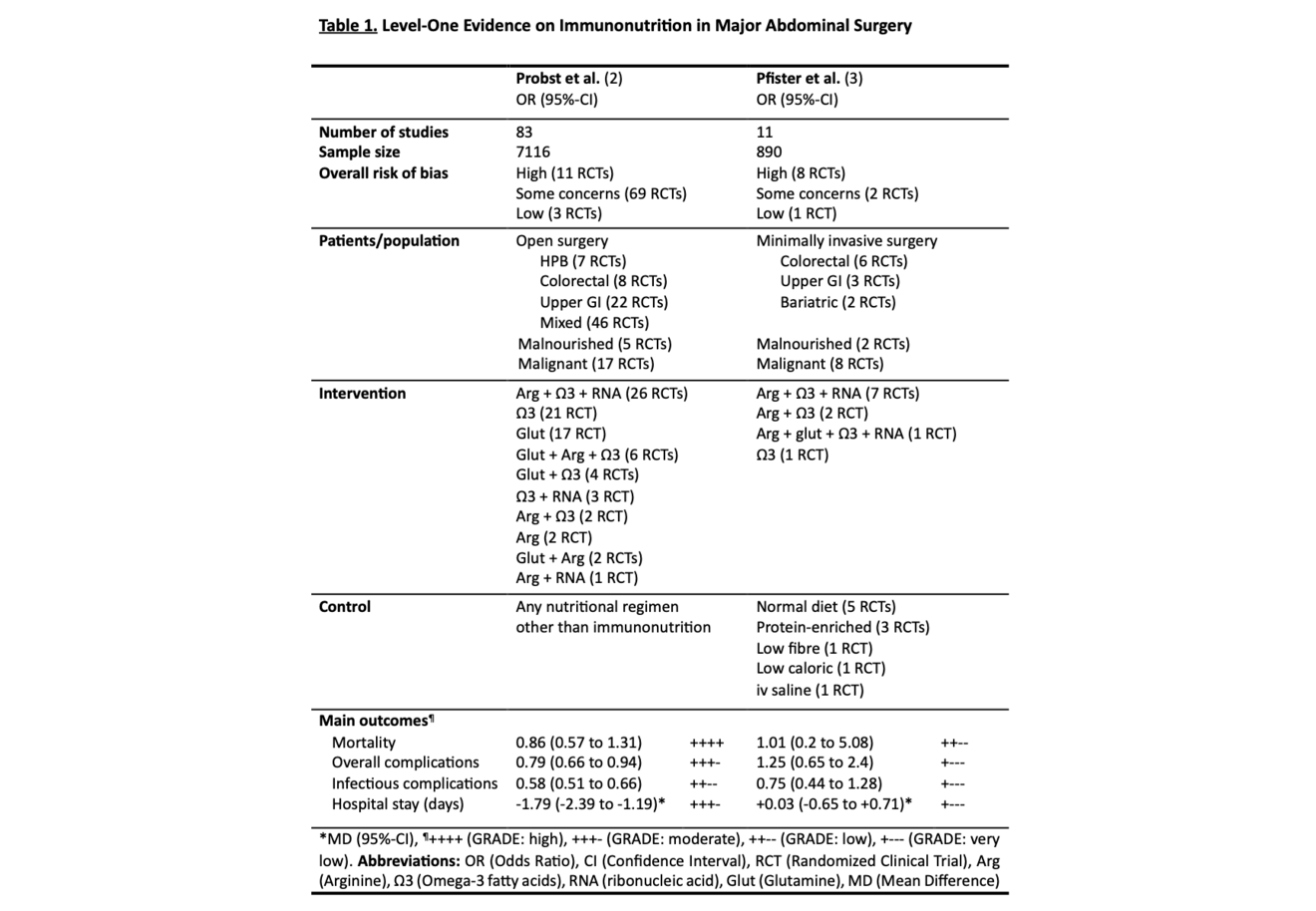
In 2017, Probst and colleagues conducted the to date largest meta-analysis of RCTs investigating immunonutrition in major abdominal surgery (2). All studies published between 1985 and 2015 comparing any combination of immunomodulatory substances to standard nutritional regimens in major abdominal surgery and reporting at least one clinically relevant endpoint (mortality, overall complications, infectious complications, hospital stay) were included. Major abdominal surgery was defined as procedures including gastrointestinal anastomosis or parenchymal transection of liver or pancreas. Gynecological and urological surgeries were excluded. A total of 83 RCTs and 7116 patients were analyzed covering hepato-pancreato-biliary (HPB) surgery (7 studies), colorectal surgery (8 studies), upper gastrointestinal surgery (22 studies), and a mix of surgical sites (46 studies). Overall analysis included a high level of clinical heterogeneity. Pooled analysis of the overall cohort found no significant difference in mortality between immunonutrition and control (OR 0.86, 95%-CI: 0.57 to 1.31; p = 0.49; I2 = 0 %; GRADE: HIGH). This finding did not change after excluding studies at high risk of bias or when including only studies at low risk of bias. Immunonutrition was associated with fewer overall complications (OR 0.79, 95%-CI: 0.66 to 0.94; p = 0.01; I2 = 32 %; GRADE: MODERATE), fewer infectious complications (OR 0.58, 95%-CI: 0.51 to 0.66; p < 0.001; I2 = 15 %; GRADE: LOW), and shorter length of stay (LOS; MD -1.79, 95%-CI: -2.39 to -1.19; p < 0.001; I2 = 82 %; GRADE: MODERATE). For the latter three outcomes, however, the effect of immunonutrition vanished when investigating trials at low risk of bias only. Of note, the overall risk of bias was low in only three of the included studies. The authors further assessed subgroups based on funding source and found the effect of immunonutrition only to be present in industry-funded studies. Grounded on these results the authors conclude that immunonutrition may be associated with reduced overall and infectious complications and shortened LOS, but the existence of significant bias lowers confidence in the evidence.
Another recent, yet unpublished, meta-analysis of RCTs (3) addressed an important and timely follow-up question: Do the potential benefits of immunonutrition observed in open major abdominal surgery persist in the era of minimally invasive approaches? Adhering to the same predefined protocol, this study synthesized evidence from 11 RCTs (n=890) comparing immunonutrition to standard nutritional regimens, therefore constituting first comprehensive level-one evidence on this topic in a minimally invasive cohort. Both laparoscopic and robotic abdominal procedures were included. The findings from this study suggest that immunonutrition results in little to no difference in postoperative mortality (OR 1.01, 95%-CI: 0.2 to 5.08; p = 0.99; I2 = 0 %; GRADE: LOW), and little to no effect on overall (OR 1.25, 95%-CI: 0.65 to 2.4; p = 0.5; I2 = 60 %; GRADE: VERY LOW) and infectious complications (OR 0.75, 95%-CI: 0.44 to 1.28; p = 0.3; I2 = 32 %; GRADE: VERY LOW), and LOS (MD +0.03 days, 95%-CI: -0.65 to +0.71; p = 0.93; I2 = 47 %; GRADE: VERY LOW), with the evidence being very uncertain for the latter three outcomes. Main reasons for downgrading the certainty of evidence were significant risk of bias, and imprecision and heterogeneity of results across studies. Importantly, the overall risk of bias was high in eight studies, with some concerns in two, and low in one. Sensitivity analysis excluding studies at high risk of bias increased the overall certainty of evidence without changing the effect on either outcome. The findings were consistent in subgroup analyses, with the evidence being very uncertain about the effect of immunonutrition on any outcome. Based on these findings, the authors conclude that the possible effects of immunonutrition in open surgery do not appear to be present in minimally invasive cohorts and acknowledge that the body of evidence remains insufficient and with marked limitations hindering reliable conclusions, particularly in nutritionally at-risk patients.
Conclusion
Despite decades of research and at times widespread clinical adoption, the promise of immunonutrition in major abdominal surgery remains largely unfulfilled. While early enthusiasm was fueled by plausible mechanistic evidence and industry-driven promise of reduced postoperative morbidity, rigorously conducted level-one evidence in open major abdominal surgery reveals that benefits on clinically relevant outcomes largely vanish in non-industry-funded studies and after excluding studies at high risk of bias. In minimally invasive major abdominal surgery—now the predominant standard of care—the alleged effects are virtually absent. What was once hyped as a key adjunct in perioperative care may only hold little hope for abdominal surgery patients. Until robust, investigator-initiated trials in clearly defined high-risk cohorts demonstrate convincing clinically meaningful benefit, the routine use of immunonutrition remains unjustified, particularly in contemporary minimally invasive surgical cohorts.
- Calder PC. Immunonutrition in surgical and critically ill patients. Br J Nutr. 2007;98 Suppl 1:S133-9.
- Probst P, Ohmann S, Klaiber U, Hüttner FJ, Billeter AT, Ulrich A, et al. Meta-analysis of immunonutrition in major abdominal surgery. Br J Surg. 2017;104(12):1594-608.
- Pfister M, von Massow N, Kalkum E, Antony P, Dullenkopf A, Hauswirth F, et al. Immunonutrition in Minimally Invasive Major Abdominal Surgery: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. 2025. Accepted for publication.
- Schindler K, Pichard C, Sulz I, Volkert D, Streicher M, Singer P, et al. nutritionDay: 10 years of growth. Clin Nutr. 2017;36(5):1207-14.
- Weimann A, Braga M, Carli F, Higashiguchi T, Hübner M, Klek S, et al. ESPEN practical guideline: Clinical nutrition in surgery. Clin Nutr. 2021;40(7):4745-61.
- Lobo DN, Gianotti L, Adiamah A, Barazzoni R, Deutz NEP, Dhatariya K, et al. Perioperative nutrition: Recommendations from the ESPEN expert group. Clin Nutr. 2020;39(11):3211-27.
- Simonsen C, de Heer P, Bjerre ED, Suetta C, Hojman P, Pedersen BK, et al. Sarcopenia and Postoperative Complication Risk in Gastrointestinal Surgical Oncology: A Meta-analysis. Ann Surg. 2018;268(1):58-69.
- Yu J, Yuan A, Liu Q, Wang W, Sun Y, Li Z, et al. Effect of preoperative immunonutrition on postoperative short-term clinical outcomes in patients with gastric cancer cachexia: a prospective randomized controlled trial. World J Surg Oncol. 2024;22(1):101.
- Miller LJ, Douglas C, McCullough FS, Stanworth SJ, Calder PC. Impact of enteral immunonutrition on infectious complications and immune and inflammatory markers in cancer patients undergoing chemotherapy: A systematic review of randomised controlled trials. Clin Nutr. 2022;41(10):2135-46.
- Ma M, Zheng Z, Zeng Z, Li J, Ye X, Kang W. Perioperative Enteral Immunonutrition Support for the Immune Function and Intestinal Mucosal Barrier in Gastric Cancer Patients Undergoing Gastrectomy: A Prospective Randomized Controlled Study. Nutrients. 2023;15(21).