Patient Blood Management is a comprehensive, evidence-based perioperative concept to detect and treat preoperative anemia and iron deficiency, to limit operative blood loss and to minimize red blood cell (RBC) transfusions using restrictive transfusion triggers and drugs to boost RBC production postoperatively (Table 1) (1).
Table 1: Patient Blood Management – The 3 pillar model
Pillar 1: Preoperative
Correct anemia / iron deficiency and stop anti-coagulation / dual platelet inhibition
– Iron (iv) + epoetin alfa (sc) + vitamin B12 (sc) + folic acid (oral)
– Stop anti-coagulation and dual platelet inhibition in time before surgery
Pillar 2: Perioperative
Reduce RBC loss
– Meticulous surgical technique
– Cell salvage and re-transfusion also in cancer surgery with irradiation of RBC suspension
– Acute normovolemic hemodilution
– Avoid coagulopathy by ROTEM / TEG based monitoring and individualized treatment according to a coagulation algorithm using anti-fibrinolytics, fibrinogen, F XIII, PCC
– Tolerate low hemoglobin values (restrictive transfusion triggers)
Pillar 3: Postoperative
Optimize anemia management
–Tolerate low hemoglobin values (restrictive transfusion triggers)
–Iron (iv) + epoetin alfa (sc) + vitamin B12 (sc) + folic acid (oral) also postoperatively
–Use elevated FiO2
Modified according to Spahn et al (1).
iv = intravenous, sc = subcutaneous, ROTEM = rotational thrombelastometry, TEG = thrombelastography, F XIII = coagulation factor XIII, PCC = prothrombin complex concentrate, FiO2 = fraction of inspired oxygen.
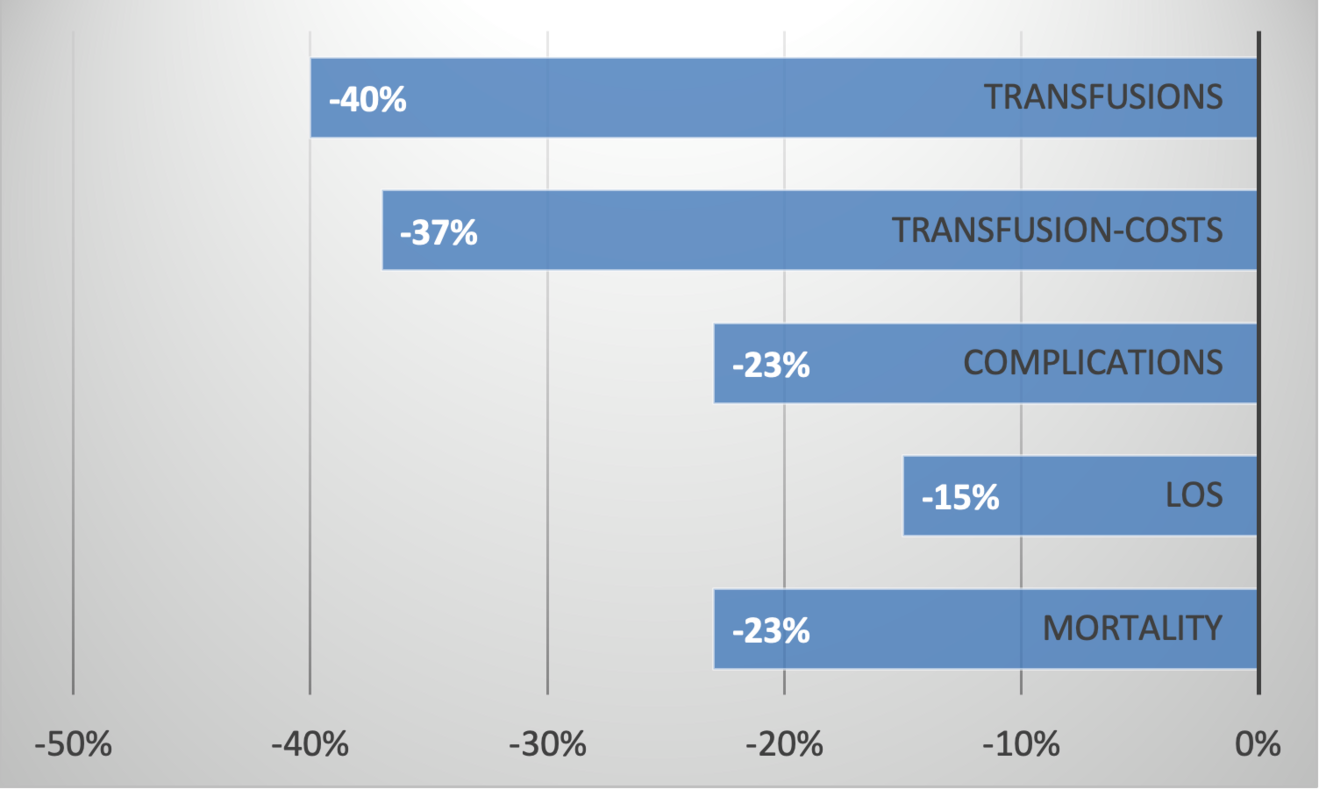
Patient Blood Management provides great benefits: Less transfusions, less complications, lower mortality, shorter length of hospital stay and major cost savings (Figure 1) (2-5). Patient Blood Management is endorsed and demanded by the World Health Organization to improve patient outcomes, patient safety and quality of care (6, 7).
Implementation – practical aspects
When you start a Patient Blood Management program at your institution, it is key to define those operations with a blood loss of more than 500 ml. It is this group of patients, the so-called focus group, in which preoperative anemia and iron deficiency detection and treatment is mandatory (1). These operations are marked in the background of the electronic operation room (OR) scheduling program. When a surgeon then announces an operation, the electronic OR- scheduling program automatically recognizes that this operation is a focus operation and notifies the preanesthetic ambulance. There, the responsible person, ideally a physician Patient Blood Manager, analyses the case. This starts with the baseline lab values of hemoglobin (Hb), ferritin, transferrin saturation (TSAT), creatinine / estimated glomerular filtration rate (eGFR) and C-reactive protein (CRP). These five simple and cheap lab values allow the broad categorization of the type of anemia and iron deficiency (if present) and allow the planning of the treatment (Table 2).
Table 2: Iron deficiency and types of preoperative anemia
Isolated iron deficiency
Hb ≥ 130 g/l, ferritin < 100 ng/ml or TSAT < 20%, CRP < 5 mg/l, eGFR = normal
Treatment: Iron iv*
Iron deficiency anemia
Hb < 130 g/l, ferritin < 100 ng/ml or TSAT < 20%, CRP < 5 mg/l, eGFR = normal
Treatment: Iron iv*, vitamin B12 1 mg sc (once) and oral folic acid 5 mg/d until surgery
Renal anemia
Hb < 130 g/l, ferritin > 100 ng/ml, TSAT > 20%, CRP < 5 mg/l, eGFR < 50 ml/min
Treatment: sc epoetin alfa (40’000 units), iron iv*, vitamin B12 1 mg sc (once) and oral folic acid 5 mg/d until surgery
Anemia of inflammation / chronic disease
Hb < 130 g/l, ferritin > 100 ng/ml, TSAT < 20%, CRP > 5 mg/l, eGFR = normal
Treatment: sc epoetin alfa (40’000 units), iron iv*, vitamin B12 1 mg sc (once) and oral folic acid 5 mg/d until surgery
* Dosing according to Ganzoni-Formula (Dose (mg) = Body weight (kg) x Hb-Delta (g/dl) x 0.24 + 500 (mg)), administration according to the information of the Compendium of the used iv iron formulation.
Hb = hemoglobin, TSAT = transferrin saturation, CRP = C-reactive protein, eGFR = estimated glomerular filtration rate, iv = intravenous, sc = subcutaneous.
The aim of the treatment is the alleviation of iron deficiency and achieving a Hb ≥ 130 g/l before surgery in men and women (8). An equal preoperative Hb is important since female patients on average are smaller, have a lower body weight, and lower blood volume per body weight and thus enter surgery with a 28% smaller RBC mass (8, 9).
Ideally, the surgeon assesses Hb, ferritin, TSAT, creatinine / eGFR and CRP either during the preoperative evaluation process or latest when the patient provides informed consent. This allows timely initiation of treatment for iron deficiency and anemia, when needed. Such treatment is often needed: 25-40% of all patients scheduled for major surgery are are anemic (10, 11), and preoperative anemia remains the leading cause of perioperative RBC transfusion (10, 12-14).
Type of preoperative anemia and specific treatment
Elective operations with a blood loss of more than 500 ml are typically cardiac operations, intraabdominal cancer operations, revision arthroplasties (hip and knee), multilevel spine and sarcoma operations. Etiology and type of anemia vary widely in these operations and only the full panel of lab values (Hb, ferritin, TSAT, creatinine / eGFR and CRP) allows the categorization necessary to choose the individually correct anemia treatment. While in the entire world population, iron deficiency is the most prevalent type of anemia (15), this is not the case in these operations in the Western world. CRP values > 5 mg/l indicative if an inflammatory condition are present preoperatively in approximatively 33% in revision arthroplasties (16), 45% in cardiac surgery (17), and 50% in the above mentioned cancer operations (18, 19). This implies that in these cases anemia of inflammation is present which is characterized by a reduced iron bioavailability (TSAT < 20%) with increase stored iron (ferritin > 100 ng/ml) and a reduced endogenous erythropoietin concentration and effectivity (20). Accordingly, the appropriate treatment is a combination of epoetin alfa and iv iron (Table 2) (20). Intravenous iron alone in this situation would not increase the Hb concentration. This extra iron would be shifted into ferritin in hepatic and reticulo-endothelial cell due to the enhanced hepcidin level (21).
Whenever we plan to treat a patient with epoetin alfa (or any other erythropoiesis stimulating agent), we need to check the thrombo-embolic risk of the patient. Did the patient suffer from a thrombo-embolic event in the last weeks? Is a (partial) thrombosis of the portal vein known in liver surgery? Is the current coagulation profile hypercoagulable (fibrinogen > 6 g/l, platelet count > 500 G/l)? Hypercoagulation is relatively frequent in these patients since inflammation upregulates also fibrinogen and platelets production (22). It is not one single number or fact that characterizes the thrombo-embolic risk of a patient but the totality of the above aspects. Nevertheless, we need to be aware that the preoperative treatment of anemia with erythropoiesis stimulating agent decreased mortality by 49% and thromboembolic complications by 43% in a near 20’000 patient cohort undergoing cardiovascular and non-cardiac operations (23).
The time course of the different treatments is interesting. Intravenous iron eliminates iron deficiency immediately. Following the administration of iv iron, sc vitamin B12 and oral folic acid, the speed of the Hb-increases depends on the severity of the underlying iron deficiency. The lower the iron parameter are, the faster is the Hb-increase. In these patients, it may be advisable to repeat laboratory tests after 3-4 weeks to assess whether target values have been reached or if a further course of treatment is required. The treatment with epoetin alfa, intravenous iron, subcutaneous vitamin B12 and oral folic acid increases the Hb levels by an average 10 g/week over 3 week period. Ideally, these 3 weeks should occur prior to surgery to allow optimal Hb-recovery. However, even initiating treatment as late as before surgery has been shown to reduce the need for RBC-transfusions (24).
Whenever we boost RBC production in anemia treatment, we generate an increased demand for vitamin B12 and folic acid. Therefore, a one-time treatment of 1 mg sc. vitamin B12 and 5 mg oral folic acid daily until surgery are initiated without plasma concentration measurement of holotranscobolamin or folic acid.
Other Patient Blood Management measures
Intraoperatively, there are additional Patient Blood Management measures that are key for a favorable patient outcome. The very most important is meticulous surgical techniques with exact hemostasis. The use of cell salvage is also important and should be considered in most major surgeries, including oncologic procedures. With the irradiation of the RBC-suspension prior to re-transfusion this is a very safe procedure and highly efficacious. Perioperative coagulation monitoring with ROTEM or TEG is also of paramount relevance, in conjunction with a coagulation factor-based coagulation algorithm. This enables individualized, goal-directed therapy and helps avoid the unnecessary administration of plasma and platelet products, resulting in shorter intensive care unit stays and reduced mortality. (25).
Cost savings
The overall savings of Patient Blood Management are likely considerably higher than the reduction in transfusion costs (Figure 1).
The reduction in postoperative complications by 23% and the shorter hospitalization (-15%) will significantly contribute to the overall cost saving.
Patient Blood Management is focused but not limited to elective surgery. Most measures can also be used in trauma and all emergency surgery (1).
Professional help for Patient Blood Management implementation
The implementation of Patient Blood Management appears to be simple, but daily practice shows that this is not always the case. Alliance Rouge in Berne (https://alliance-rouge.ch/) offers structured support for the implementation including e-learning and template forms for most of the above procedures and measures.
Conflicts of interest
GHS reports having received honoraria from CSL Vifor (Switzerland) Villars-sur-Glâne, Switzerland and CSL Vifor (International), St. Gallen, Switzerland, Switzerland for lecturing.
DRS reports being the chair of the ABC-Trauma Faculty, sponsored by unrestricted educational grants from Alexion Pharma Germany GmbH, Munich, Germany, CSL Behring GmbH, Marburg, Germany, and LFB Biomédicaments, Courtaboeuf Cedex, France. He is also the president of Alliance Rouge, Bern, Switzerland, CEO of Swiss-PBM-Consulting GmbH, Zurich, Switzerland and a member of the Advisory Board of Saipient AG, Zurich, Switzerland.
He received honoraria / travel support for consulting or lecturing from:
Alliance Rouge, Bern, Switzerland, Danube University of Krems, Austria, European Society of Anesthesiology and Intensive Care, Brussels, BE, International Foundation for Patient Blood Management, Basel, Switzerland, Korean Society of Anesthesiologists, Seoul, Korea, Network for the Advancement of Patient Blood Management, Haemostasis and Thrombosis, Paris, France, Society for the Advancement of Blood Management, Mount Royal NJ, Alexion Pharmaceuticals Inc., Boston, MA, AstraZeneca AG, Baar, Switzerland, Baxter AG, Glattpark, Switzerland, Bayer AG, Zürich, Switzerland, B. Braun Melsungen AG, Melsungen, Germany, CSL Behring GmbH, Hattersheim am Main, Germany and Berne, Switzerland, CSL Vifor (Switzerland) Villars-sur-Glâne, Switzerland, CSL Vifor (International), St. Gallen, Switzerland, Celgene International II Sàrl, Couvet, Switzerland, Daiichi Sankyo AG, Thalwil, Switzerland, Haemonetics, Braintree, MA, USA, iSEP, Nantes, France, Novo Nordisk Health Care AG, Zurich, Switzerland, Octapharma AG, Lachen, Switzerland, Pharmacosmos A/S, Holbaek, Denmark, Pierre Fabre Pharma, Alschwil, Switzerland, Portola Schweiz GmbH, Aarau, Switzerland, Roche Diagnostics International Ltd, Reinach, Switzerland, Shire Switzerland GmbH, Zug, Switzerland, Werfen, Bedford, MA, Zuellig Pharma Holdings, Singapore, Singapore.
- Spahn DR, Munoz M, Klein AA, Levy JH, Zacharowski K. Patient Blood Management: Effectiveness and Future Potential. Anesthesiology. 2020;133(1):212–22.
- Kaserer A, Rossler J, Braun J, Farokhzad F, Pape HC, Dutkowski P, et al. Impact of a Patient Blood Management monitoring and feedback programme on allogeneic blood transfusions and related costs. Anaesthesia. 2019;74(12):1534–41.
- Althoff FC, Neb H, Herrmann E, Trentino KM, Vernich L, Fullenbach C, et al. Multimodal Patient Blood Management Program Based on a Three-pillar Strategy: A Systematic Review and Meta-analysis. Annals of surgery. 2019;269(5):794–804.
- Leahy MF, Hofmann A, Towler S, Trentino KM, Burrows SA, Swain SG, et al. Improved outcomes and reduced costs associated with a health-system-wide patient blood management program: a retrospective observational study in four major adult tertiary-care hospitals. Transfusion. 2017;57(6):1347–58.
- Garcia-Casanovas A, Bisbe E, Vizoso A, Sarsanedas E, Garcia-Altes A, Colomina MJ, et al. Association between Adherence to Patient Blood Management Recommendations and Postoperative Complications in Hip and Knee Arthroplasty. Anesthesiology. 2025;143(1):24–37.
- WHO-1. Policy Brief: The urgent need to implement Patient Blood Management. https://appswhoint/iris/bitstream/handle/10665/346655/9789240035744-engpdf. 2021.
- WHO-2. Guidance on implementing patient blood management to improve global blood health status. https://wwwwhoint/publications/i/item/9789240104662 2025.
- Kaserer A, David Mazer C, Braun J, Spahn DR. Achieving a preoperative haemoglobin above 130 g L(-1) may be more important in female than in male patients before cardiac surgery. British journal of anaesthesia. 2023;131(4):636–8.
- Cavalli LB, Pearse BL, Craswell A, Anstey CM, Naidoo R, Rapchuk IL, et al. Determining sex-specific preoperative haemoglobin levels associated with intraoperative red blood cell transfusion in cardiac surgery: a retrospective cohort study. British journal of anaesthesia. 2023;131(4):653–63.
- Judd L, Hof L, Beladdale L, Friederich P, Thoma J, Wittmann M, et al. Prevalence of pre-operative anaemia in surgical patients: a retrospective, observational, multicentre study in Germany. Anaesthesia. 2022;77(11):1209–18.
- Lasocki S, Belbachir A, Mertes PM, Pelley EL, Capdevila X. Evaluation of Anemia and Iron Deficiency in French Surgical Departments: The National Multicenter Observational PERIOPES Study. Anesthesia and analgesia. 2023;137(1):182–90.
- Skorupski CP, Cheung MC, Hallet J, Kaliwal Y, Nguyen L, Pavenski K, et al. Preoperative Anemia and Iron Deficiency in Elective Gastrointestinal Cancer Surgery Patients. J Surg Oncol. 2025;131(4):614–23.
- LaPar DJ, Hawkins RB, McMurry TL, Isbell JM, Rich JB, Speir AM, et al. Preoperative anemia versus blood transfusion: Which is the culprit for worse outcomes in cardiac surgery? The Journal of thoracic and cardiovascular surgery. 2018;156(1):66–74 e2.
- Warner MA, Hanson AC, Schulte PJ, Sanz JR, Smith MM, Kauss ML, et al. Preoperative Anemia and Postoperative Outcomes in Cardiac Surgery: A Mediation Analysis Evaluating Intraoperative Transfusion Exposures. Anesthesia and analgesia. 2024;138(4):728–37.
- Collaborators GBDA. Prevalence, years lived with disability, and trends in anaemia burden by severity and cause, 1990-2021: findings from the Global Burden of Disease Study 2021. Lancet Haematol. 2023;10(9):e713–e34.
- Piper KE, Fernandez-Sampedro M, Steckelberg KE, Mandrekar JN, Karau MJ, Steckelberg JM, et al. C-reactive protein, erythrocyte sedimentation rate and orthopedic implant infection. PLoS One. 2010;5(2):e9358.
- Rossler J, Hegemann I, Schoenrath F, Seifert B, Kaserer A, Spahn GH, et al. Efficacy of quadruple treatment on different types of pre-operative anaemia: secondary analysis of a randomised controlled trial. Anaesthesia. 2020;75(8):1039–49.
- Bath M, Viveiros A, Schaefer B, Klein S, Pammer LM, Wagner S, et al. Impact of preoperative anemia, iron-deficiency and inflammation on survival after colorectal surgery-A retrospective cohort study. PLoS One. 2022;17(7):e0269309.
- Fuglestad AJ, Meltzer S, Ree AH, McMillan DC, Park JH, Kersten C. The clinical value of C-reactive protein and its association with tumour location in patients undergoing curative surgery for colorectal cancer - a ScotScan collaborative study. Acta Oncol. 2022;61(10):1248–55.
- Weiss G, Ganz T, Goodnough LT. Anemia of inflammation. Blood. 2019;133(1):40–50.
- Auerbach M, DeLoughery TG, Tirnauer JS. Iron Deficiency in Adults: A Review. JAMA. 2025;333(20):1813–23.
- Mantovani A, Garlanda C. Humoral Innate Immunity and Acute-Phase Proteins. The New England journal of medicine. 2023;388(5):439–52.
- Choi UE, Nicholson RC, Frank SM, Cha S, Cho BC, Lawton JS, et al. Use of preoperative erythropoietin-stimulating agents is associated with decreased thrombotic adverse events compared to red blood cell transfusion in surgical patients with anaemia. Vox sanguinis. 2024;119(11):1174–82.
- Spahn DR, Schoenrath F, Spahn GH, Seifert B, Stein P, Theusinger OM, et al. Effect of ultra-short-term treatment of patients with iron deficiency or anaemia undergoing cardiac surgery: a prospective randomised trial. Lancet. 2019;393(10187):2201–12.
- Stein P, Kaserer A, Sprengel K, Wanner GA, Seifert B, Theusinger OM, et al. Change of transfusion and treatment paradigm in major trauma patients. Anaesthesia. 2017;72(11):1317–26.