Perihilar cholangiocarcinoma (pCCA) remains among the most challenging solid tumors to treat and is often associated with a dismal prognosis 1. Only a minority of patients are amenable to surgery, which is the only chance for cure. Patients undergoing resection face up to 70 % major complications (Clavien-Dindo 2,3 ≥ IIIa) and 90-day mortality of 13 %, even in a low-risk benchmark setting 1,4. This disease complexity combined with a lack of high-level data due to its low incidence result in a lack of consensus on many aspects of disease management. For example, there are still no uniformly accepted resectability criteria 5-7, and also the optimal preoperative route and timing of biliary drainage remains a matter of debate, resulting in suboptimal patient management 8. Matters are further complicated with novel multimodal treatment strategies, including neoadjuvant chemo-immunotherapy, and the rise of transplant oncology. Therefore, the Consensus4pCCA intiative – an international expert consensus meeting held in Milan in December 2024 – aimed to critically assess the body of evidence on the management of pCCA and to provide comprehensive, evidence-based recommendations on the multidisciplinary management of pCCA 9.
Guideline Development
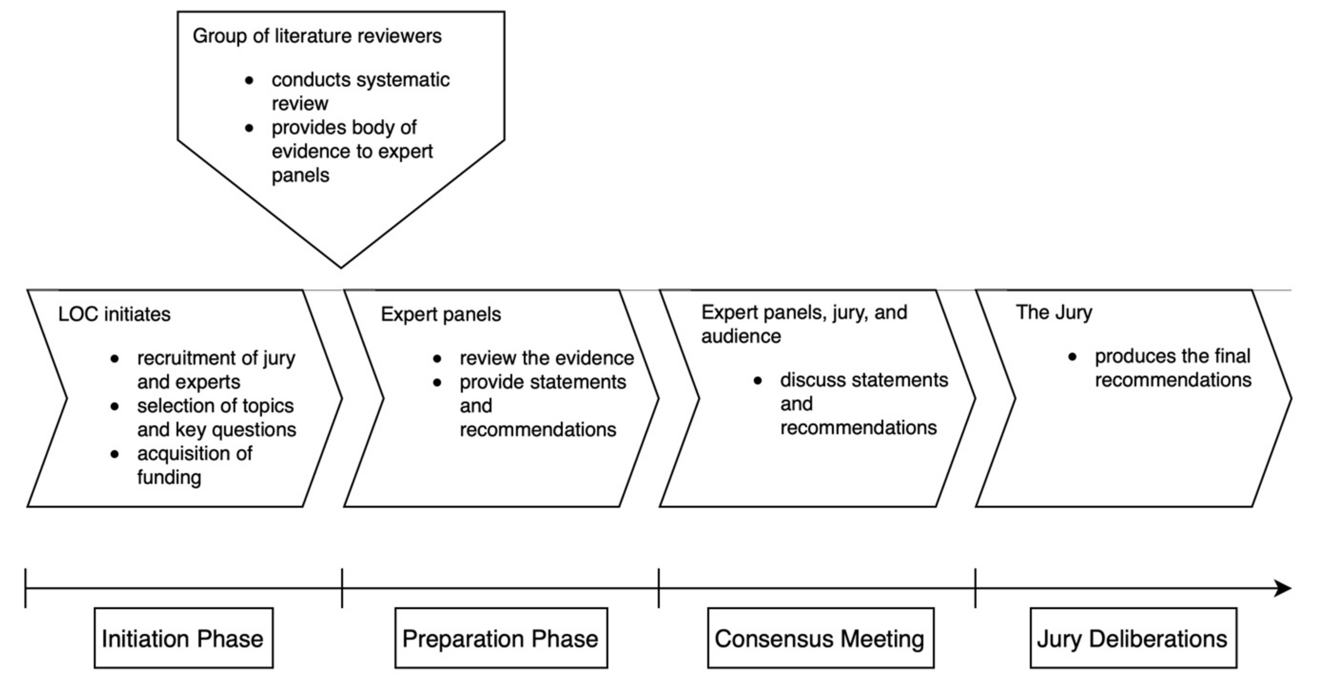
The Zurich-Danish model
The development of evidence-based consensus and recommendations adhered to the Zurich-Danish format 10 (Figure 1).
This model has been validated in numerous international consensus initiatives on for example hepatocellular carcinoma 11, surgical quality 12, and robotic hepato-pancreato-biliary surgery 13. It consists of three phases: 1. Preparation phase, 2. Consensus meeting, 3. Jury deliberations. Importantly, the work of the experts in the field and the jury are strictly separated to develop unbiased recommendations.
Preparation phase
A scientific steering committee (SC) together with the organizing committee (OC) defined 8 panels covering all aspects of disease management: 1. Definition and diagnosis, 2. Perioperative planning and surgical resectability, 3. Preoperative optimization, 4. Surgery, 5. Morbidity and mortality, 6. Perioperative oncological treatments, 7. Transplantation, and 8. Expected outcomes. For each panel, the SC and OC defined 5-7 clinical questions. In the preparation phase several months prior to the consensus meeting, expert panels – chosen based on their widely recognized expertise and scientific work on a given topic – were tasked to answer the predefined clinical questions and propose recommendations based on the highest-quality evidence. For each panel, a designated panel chair coordinated the panel’s work. A systematic literature review conducted by a literature working group preceded this step to provide the experts with the relevant literature.
Consensus meeting
The in-person consensus meeting was held in Milan on December 5-6, 2024. The respective panel chairs presented their panel’s recommendations and scientific rationale. Each presentation was followed by an open discussion first among the panelists and jury, then including also the public audience on site.
Jury deliberations
The meeting sessions were followed by closed jury deliberations. The impartial jury was tasked to critically review the proposed recommendations with the option to summon and further interrogate the expert panel chairs. The jury was exclusively responsible for adopting the final recommendations based on the GRADE approach 14,15.
Recruitement of experts and the jury
Experts had to fulfil at least one of the following criteria: 1. Academic track record in the field, 2. Exceptional clinical expertise in the management of pCCA, 3. Cholangiocarcinoma patient representant. All different stakeholders involved in disease management were represented in the different panels: surgeons, gastroenterologists, interventional radiologists, medical oncologists, and pathologists. Also, great care was taken to maintain a geographical and demographical balance.
The ten members of the jury included non-hepato-pancreato-biliary surgeons, hepatologists, epidemiologists, and bioethicists. Importantly, none of the members were allowed to be directly involved with pCCA management or research to avoid potential bias. They were chosen based on their expertise in guideline development and its methodology.
Systematic literature review
A dedicated group of 17 literature reviewers conducted a systematic assessment of the body of evidence. The review adhered to SIGN50 guidelines 16. Three databases (Embase, Medline, and Cochrane) were systematically searched. Original articles investigating any aspect of pCCA management were eligible. According to SIGN50 guidelines, studies were classified as of either high, acceptable, low, or unacceptable quality. Studies of unacceptable quality were excluded. The final database comprising the highest available evidence in the field was then provided to the expert panelists in the preparation phase.
Grading of recommendations
The level of evidence for each recommendation was assessed using the GRADE approach (levels 1-5). Recommendations were graded strong or weak taking the following factors into account: certainty of evidence, balance between desirable and undesirable effects, confidence in the magnitude of effect, values and preferences and their variability, ethical obligations, feasibility, and resource use 14,15,17.
Final Recommendations
A total of 570 records were included through the systematic literature review and were considered for consensus development by 71 international experts (56 % Europe, 24 % North America, 17 % Asia, 3 % South America). Based on this information, the jury adopted a total of 71 detailed statements and recommendations (available in the published article 9), comprehensively covering the different aspects of multidisciplinary disease management.
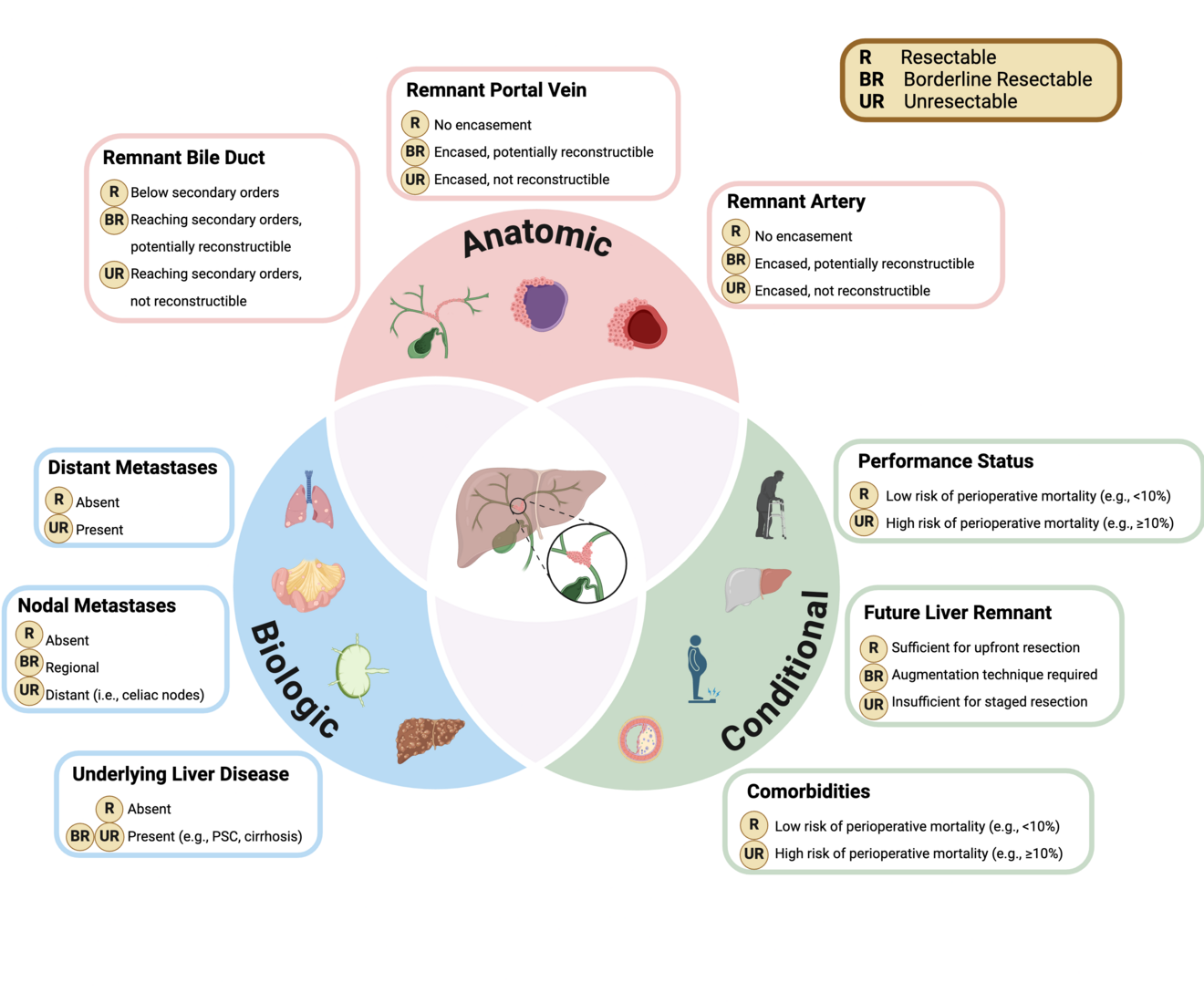
Key take-aways were that harmonizing treatment pathways across specialties and institutions, making patients amenable to high-quality surgery as early as possible, and reducing its associated complications must be of highest priority. One specific highlight was the proposal of comprehensive resectability criteria (Figure 2).
For the first time in this disease, experts proposed to determine surgical resectability based on anatomical, biologic, and conditional factors. These criteria require further validation. Importantly, assessment of anatomical resectability should be based on high-quality cross-sectional imaging performed prior to any biliary intervention, well-known to cause image artifacts and thus misinterpretation. The future liver remnant – one key determinant of resectability – should be assessed using standardized formulae. Recommended cutoffs are > 30 % in healthy parenchyma, and > 40 % in frail or comorbid patients, or those with cholestasis/cholangitis or malnutrition. Also, the different techniques for liver hypertrophy were intensely discussed, with the jury finally endorsing portal-vein embolization as the primary approach, and liver venous deprivation as an alternative in anticipated impaired regenerative capacity or the need for extended augmentation (e.g., future liver remnant < 20 % in healthy and < 25 % in altered parenchyma, right extended hepatectomy). Another heated debated evolved around the role of liver transplantation for pCCA, and particularly adequate patient selection. Transplant candidates primarily base on the validated Mayo criteria and currently still predominantly focus on unresectable disease. The role of liver transplantation in resectable disease remains a matter of debate. While the jury acknowledged a potential benefit, such strategy must be weighed against limited access to liver grafts due to global organ shortage.
Finally, and propably most importantly, the jury defined areas that need further attention and should be subject to future research priorities. These primarily include the implemenation of high-quality, large scale, national and international registries, and emphasize the absolute need for standardized outcome reporting, including parameters of clinical relevance and patient-centered outcomes.
Conclusions
The Consensus4pCCA represents a token of multidisciplinary, international collaboration aiming at harmonizing management strategies of complex disease like pCCA. Such efforts play a significant role, particularly in highly-specialized, low-incidence disease with a limited body of evidence and marked practice variation worldwide. Its robust methodology strictly separating experts from jury is paramount to avoid opinionated guidance and ensure impartial recommendations across institutional and geographical borders. Ultimately, recommendations must translate into meaningful change in clinical practice and alignment of research efforts to result in sustained improvement of patient care.
- Franken LC, Schreuder AM, Roos E, et al. Morbidity and mortality after major liver resection in patients with perihilar cholangiocarcinoma: A systematic review and meta-analysis. Surgery. May 2019;165(5):918-928. doi:10.1016/j.surg.2019.01.010
- Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. Aug 2004;240(2):205-13. doi:10.1097/01.sla.0000133083.54934.ae
- Clavien PA, Barkun J, de Oliveira ML, et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. Aug 2009;250(2):187-96. doi:10.1097/SLA.0b013e3181b13ca2
- Mueller M, Breuer E, Mizuno T, et al. Perihilar Cholangiocarcinoma - Novel Benchmark Values for Surgical and Oncological Outcomes From 24 Expert Centers. Ann Surg. Nov 01 2021;274(5):780-788. doi:10.1097/SLA.0000000000005103
- Otto CC, Mantas A, Heij LR, et al. Preoperative predictors for non-resectability in perihilar cholangiocarcinoma. Article. World Journal of Surgical Oncology. 2024;22(1)doi:10.1186/s12957-024-03329-1
- Pratt CG, Whitrock JN, Shah SA, Fong ZV. How to Determine Unresectability in Hilar Cholangiocarcinoma. Surg Clin North Am. Feb 2024;104(1):197-214. doi:10.1016/j.suc.2023.09.001
- Nagino M, Ebata T, Yokoyama Y, et al. Evolution of surgical treatment for perihilar cholangiocarcinoma: A single-center 34-year review of 574 consecutive resections. Article. Annals of Surgery. 2013;258(1):129-140. doi:10.1097/SLA.0b013e3182708b57
- Ratti F, Marino R, Muiesan P, et al. Results from the european survey on preoperative management and optimization protocols for PeriHilar cholangiocarcinoma. HPB (Oxford). Nov 2023;25(11):1302-1322. doi:10.1016/j.hpb.2023.06.013
- Pfister M, Ratti F, Gores GJ, et al. Recommendations on Perihilar Cholangiocarcinoma. The Milan Jury-Based Consensus. Ann Surg. Jun 06 2025;doi:10.1097/SLA.0000000000006773
- Lesurtel M, Perrier A, Bossuyt PM, Langer B, Clavien PA. An independent jury-based consensus conference model for the development of recommendations in medico-surgical practice. Surgery. Mar 2014;155(3):390-7. doi:10.1016/j.surg.2013.10.003
- Clavien PA, Lesurtel M, Bossuyt PM, et al. Recommendations for liver transplantation for hepatocellular carcinoma: an international consensus conference report. Lancet Oncol. Jan 2012;13(1):e11-22. doi:10.1016/S1470-2045(11)70175-9
- Domenghino A, Walbert C, Birrer DL, Puhan MA, Clavien PA, group OMc. Consensus recommendations on how to assess the quality of surgical interventions. Nat Med. Apr 2023;29(4):811-822. doi:10.1038/s41591-023-02237-3
- Hobeika C, Pfister M, Geller D, et al. Recommendations on Robotic Hepato-Pancreato-Biliary Surgery. The Paris Jury-Based Consensus Conference. Ann Surg. May 24 2024;doi:10.1097/SLA.0000000000006365
- Andrews J, Guyatt G, Oxman AD, et al. GRADE guidelines: 14. Going from evidence to recommendations: the significance and presentation of recommendations. J Clin Epidemiol. Jul 2013;66(7):719-25. doi:10.1016/j.jclinepi.2012.03.013
- Andrews JC, Schünemann HJ, Oxman AD, et al. GRADE guidelines: 15. Going from evidence to recommendation-determinants of a recommendation's direction and strength. J Clin Epidemiol. Jul 2013;66(7):726-35. doi:10.1016/j.jclinepi.2013.02.003
- SIGN 50: A guideline developer’s handbook. http://www.sign.ac.uk/guidelines/fulltext/50/index.html
- Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. Apr 26 2008;336(7650):924-6. doi:10.1136/bmj.39489.470347.AD