Histopathologic examination of biopsies is necessary for a definitive diagnosis and important for surgical planning. Following diagnosis, surgical resection of the aganglionic intestinal segment with anastomosis of the proximal bowel to the anus is usually performed and known as the endorectal pull-through procedure [GS1] . Even though many patients may recover well and attain better bowel function after surgery, long term outcomes of Hirschsprung`s disease are far from satisfactory since constipation and incontinence can cause lifelong significant problems [4]. The most dangerous [HCS2] complication is Hirschsprung-associated enterocolitis with toxic megacolon, which can occur both preoperatively and postoperatively.
Complex, time consuming and invasive diagnosis
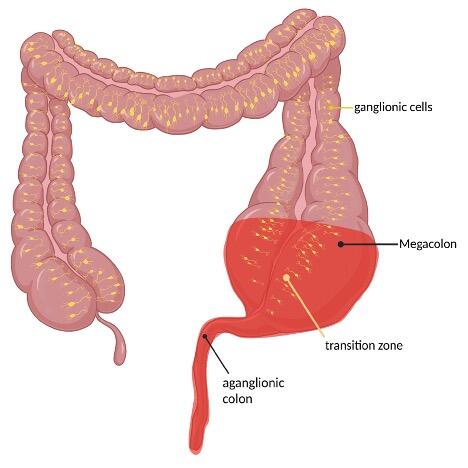
The clinical presentation of Hirschsprung`s disease can be highly variable in both symptoms and severity. 90% of infants become symptomatic during the first 24 to 72 hours of life through delayed passage of meconium [5]. Other manifestations include constipation, poor feeding, weight gain and progressive abdominal distention even firstly noted in older children. In rare cases cecal or appendiceal perforation may be the initial [5]. In case of Hirschsprung associated enterocolitis (HAEC) patients develop signs and symptoms of intestinal inflammation characterized by abdominal distension, diarrhea, fever with pain and agitation up to signs of shock in case of a toxic megacolon [3].
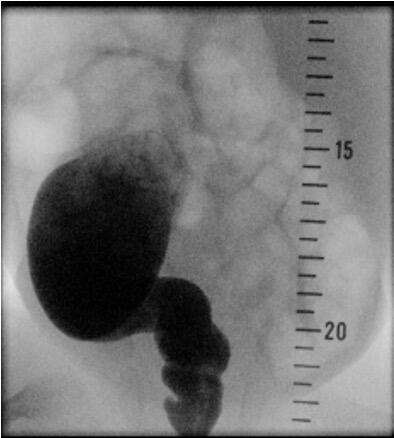
Patients showing suspicious signs for Hirschsprung`s disease can be screened with a diagnostic workup like radiological contrast enema and anorectal manometry. Ideally, a funnel-shaped transition zone appears on the radiological contrast image (Figure 2). Unfortunately, a contrast enema can be interpreted as normal in 24% of infants with Hirschsprung`s disease, and false-positive results are common, so this approach cannot be used to make a reliable diagnosis [6]. Conspicuousness in anorectal manometry is an absence of recto anal inhibitory reflex.
The gold standard for confirming the diagnosis of Hirschsprung`s disease is the histopathological evidence of absent neurons of the myenteric (Auerbach) and the submucosal (Meissner) plexus in biopsy specimens [7]. Biopsy collection can be done in two ways: An endorectal suction procedure for the assessment of the submucosal plexus preferred in neonates or open surgical full-thickness biopsies in older children. Both techniques are invasive, time-consuming and open biopsy collection requires general anesthesia [8].
Histopathologic evaluation of Hirschsprung`s disease follows. If ganglion cells are missing on conventional histopathology with hematoxyn-eosin (HE) staining, additional immunohistochemical or enzymatic examination should be considered to increase sensitivity [9]. This detects both neurons and parasympathetic nervous system activity. In experienced settings, an enzymatic examination via three oxygenases is performed: Acetylcholinesterase, lactate dehydrogenase, succinyl acid dehydrogenase or nitroxide synthase. Alternatively positive control staining with calretinin, which is a calcium-binding protein found in the intrinsic nerves of the muscularis mucosae and lamina propria [7] will be performed.
No definitive curative treatment
Initial management of Hirschsprung`s disease varies based on age of the patient, health status and the surgeon`s preference. Some physicians use scheduled home colonic saline irrigations and laxatives to regulated functional bowel obstruction. This allows the patient to grow and gain weight for several months. Others proceed to immediate, definitive surgery. In the case of establishing the diagnosis during a period of Hirschsprung associated enterocolitis, the patient might need emergency temporary colostomy proximal to the aganglionosis in a segment of normal bowel [10].
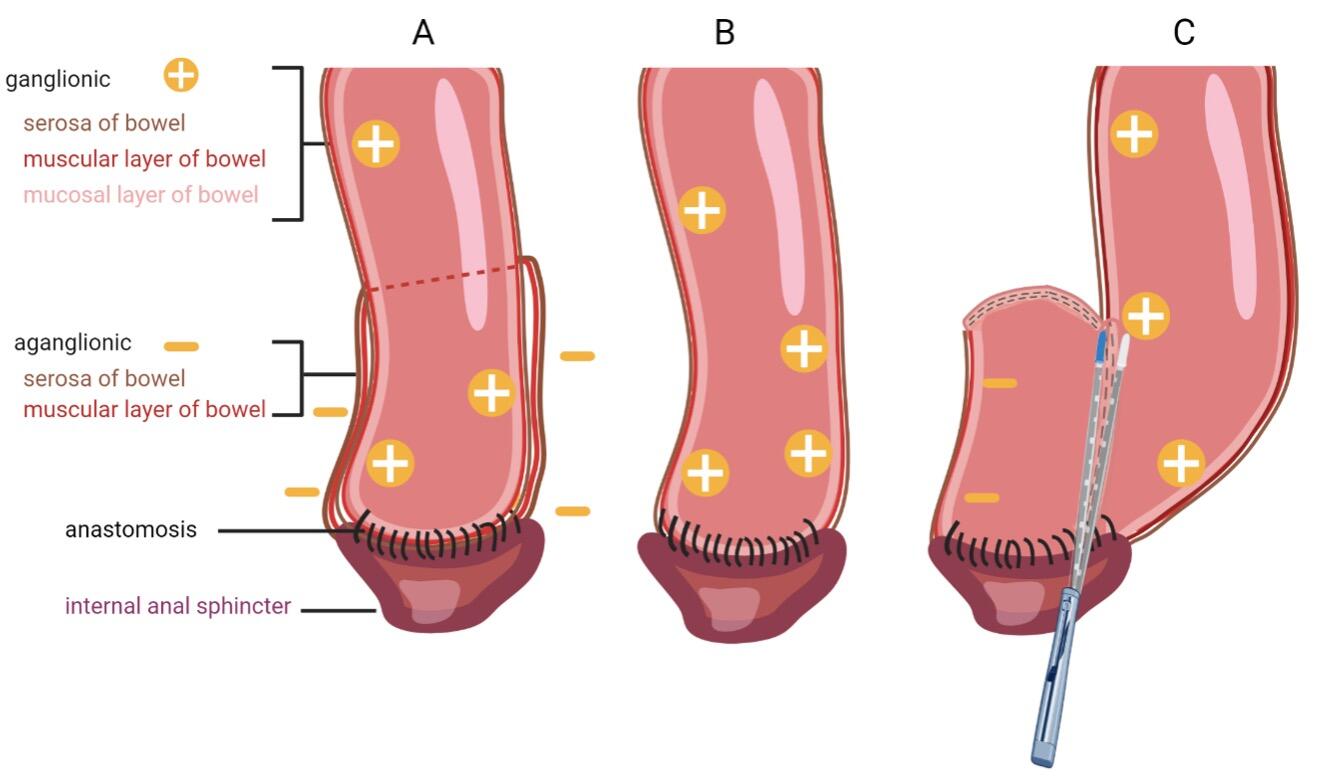
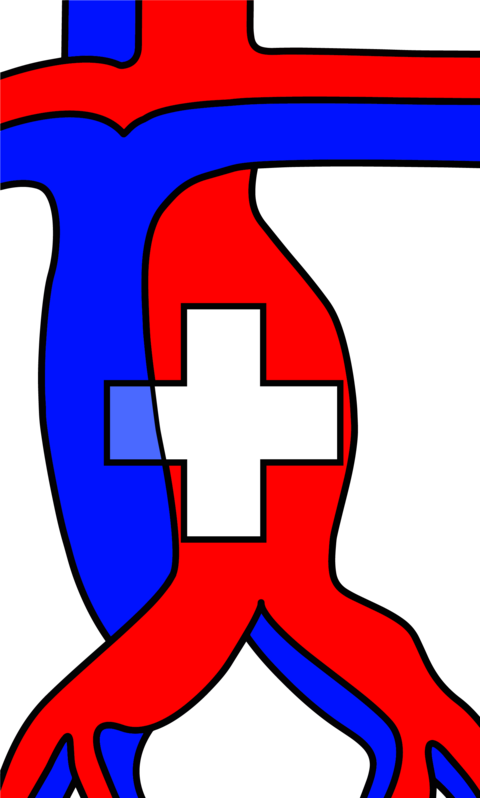
Definitive surgery requires total resection of the aganglionic intestine with anastomosis of ganglionated bowel to the anal canal within the normal innervated internal anal sphincter. There are many surgical approaches to Hirschsprung`s disease including the transabdominal approach or combined approach via abdominal laparotomy or laparoscopy and perineal approach. The three most common accomplished techniques following the principles of Swenson, Duhamel and Soave (Figure 3) [11]. Patients mostly get operated by transanal endorectal pull-through (TERPT). Modification of these techniques are proposed with the goal of minimally invasive surgery and consequently shorter operation time, shorter hospital stay and improved cosmetical outcome.
(A) In the Soave technique, the aganglionic colon and rectum are removed. To minimize pelvic nerve injury, a remaining aganglionic seromuscular “cuff” is left, through which ganglionated bowel is pulled through and connected to the anal canal. To avoid recurrent obstruction, the muscular cuff is split posteriorly.
(B) The first described Swenson procedure involves an end-to-end anastomosis of the ganglionated colon to the anal canal with nearly complete resection of the aganglionic intestinal segment [12].
(C) In the Duhamel procedure the aganglionic bowel is resected up to the entire rectum. A presacral dissection is performed and the ganglionated bowel is pulled through and anastomosed to the native rectum. This is achieved via a posterior, distal transverse enterotomy in the native rectum. The anterior part of the neorectum remains native, while the posterior portion becomes the ganglionated pull-through segment. This approach creates a larger reservoir and avoids anterior dissection and potential injury to the bladder autonomic nervous system [10].
Actually, the predominantly chosen strategy follows the modified Soave technique after De la Torre-Mondargon, also called single-stage TERP with only an anal approach, minimizing the risk of complications for abdominal adhesions, pelvic nerve injury and superior pain alleviation [12].
Ideally, after a successful surgical intervention, a child born with Hirschsprung`s disease should thrive appropriately for its age, achieve complete fecal continence, and should be cured from episodes of enterocolitis. Realistically, a significant number of patients proceed to have problems even after perfect surgery. Hirschsprung associated enterocolitis is a challenge, with high morbidity and possible mortality, depending on the clinical severity of the complication. It can occur in up to 42% of patients postoperatively undergoing TERPT [13].
Research strategies
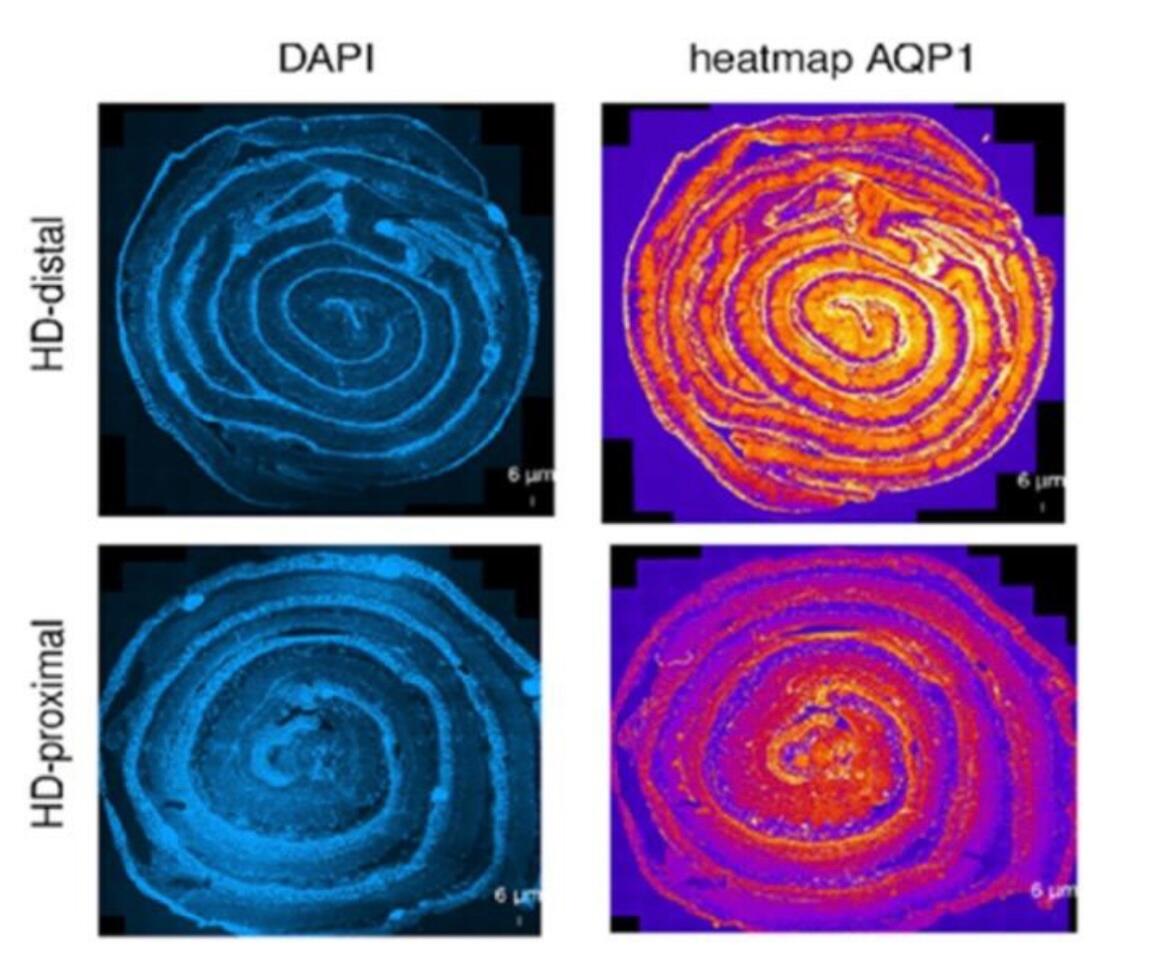
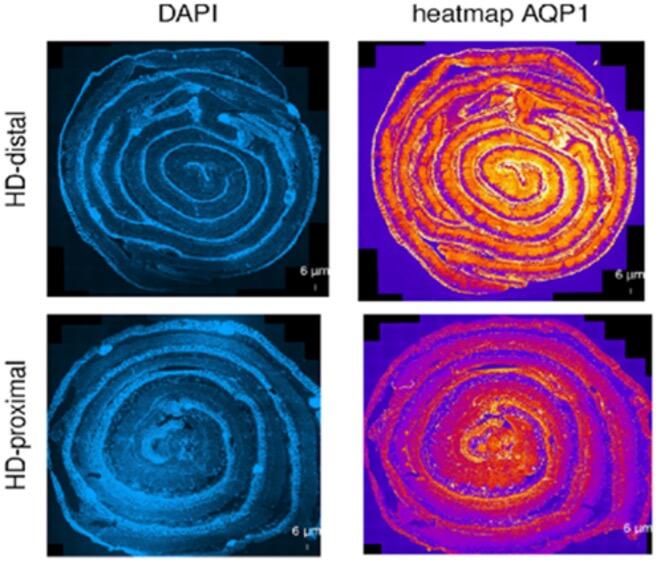
The focus of our multicentric research (cooperation in Switzerland with: Department of Pediatric Surgery, Children`s Hospital Zürich; Department of Pediatric Surgery, Kantonspital St. Gallen; Department of Pediatric Surgery, Inselspital Bern; Department of Pediatric Surgery, Hôpitaux Universitaires de Genève; in Germany with: Department of Pediatric Surgery, University Hospital Heidelberg; Department of Pediatric Surgery, Municipal Hospital Karlsruhe; Department of Pediatric Surgery, University Hospital Düsseldorf) is to understand the underlying molecular mechanisms that are associated with erroneous intestinal motility associated with Hirschsprung`s disease and HAEC. Following the study on neuro-immune interactions in the gut [14, 15], the group focuses now on how morphological aspects of the intestine interact with the functional aspects in the patient as well as on new functional models for this disease [16]. One emphasis of our research lies on intestinal water permeability which differs between the aganglionic and ganglionic parts of the colon (Figure 4). The transmembraneous water channels in the human intestine are physiologically crucial for maintaining the body homeostasis and ensure digestive and absorptive functions [17]. Changes in membrane permeability potentially have a substantial impact on the development of HAEC. Despite numerous hypotheses and proposed causes, the biological mechanisms behind this congenital disease remain poorly comprehended. Our research aims to make a positive impact by enhancing our understanding and addressing the significant clinical challenges associated with HAEC, even in the face of ongoing advancements in surgical techniques. With our research we hope to positively influence and ameliorate the massive clinical issues resulting from this congenital disease despite continuous improvement of surgical techniques.
Conclusion
Although the first description of the congenital intestinal disease dates back more than 140 years, to date there are no prognostic factors to estimate the complication rate after the surgical treatment for the affected patient. Indeed Hirschsprung`s disease represents a challenge for pediatricians, pediatric surgeons, and pediatric pathologists and could be associated with significant life restrictions for the patient. Even appropriate surgical repair requires ongoing, lifelong management regarding continence, fertility, sexual satisfaction, and quality of life, as well as continuous check-ups by general surgeons, gynecologists and urologists. The more important is the ongoing basic research to improve the knowledge on a molecular level to improve clinical outcome for children with this congenital disease.
- Klein, M. and I. Varga, Hirschsprung's Disease-Recent Understanding of Embryonic Aspects, Etiopathogenesis and Future Treatment Avenues. Medicina (Kaunas), 2020. 56(11).
- Butler Tjaden, N.E. and P.A. Trainor, The developmental etiology and pathogenesis of Hirschsprung disease. Transl Res, 2013. 162(1): p. 1-15.
- Kyrklund, K., et al., ERNICA guidelines for the management of rectosigmoid Hirschsprung's disease. Orphanet J Rare Dis, 2020. 15(1): p. 164.
- Aworanti, O.M., et al., Does Functional Outcome Improve with Time Postsurgery for Hirschsprung Disease? Eur J Pediatr Surg, 2016. 26(2): p. 192-9.
- Langer, J.C., Hirschsprung disease. Curr Opin Pediatr, 2013. 25(3): p. 368-74.
- Peyvasteh, M., et al., DIAGNOSTIC ACCURACY OF BARIUM ENEMA FINDINGS IN HIRSCHSPRUNG'S DISEASE. Arq Bras Cir Dig, 2016. 29(3): p. 155-158.
- Musa, Z.A., et al., Diagnostic roles of calretinin in hirschsprung disease: A comparison to neuron-specific enolase. Saudi J Gastroenterol, 2017. 23(1): p. 60-66.
- Ambartsumyan, L., C. Smith, and R.P. Kapur, Diagnosis of Hirschsprung Disease. Pediatric and Developmental Pathology, 2019. 23(1): p. 8-22.
- Nabi, Z., et al., Diagnosis of Hirschsprung's disease in children: Preliminary evaluation of a novel endoscopic technique for rectal biopsy. JGH Open, 2018. 2(6): p. 322-326.
- Gause, C.D. and S. Krishnaswami, Management of Anorectal Malformations and Hirschsprung Disease. Surgical Clinics of North America, 2022. 102(5): p. 695-714.
- Yan, B.L., et al., Transanal endorectal pull-through procedure versus transabdominal surgery for Hirschsprung disease: A systematic review and meta-analysis. Medicine (Baltimore), 2019. 98(32): p. e16777.
- De La Torre, L. and J.C. Langer, Transanal endorectal pull-through for Hirschsprung disease: technique, controversies, pearls, pitfalls, and an organized approach to the management of postoperative obstructive symptoms. Seminars in Pediatric Surgery, 2010. 19(2): p. 96-106.
- Ahmad, H., et al., Evaluation and Management of Persistent Problems After Surgery for Hirschsprung Disease in a Child. Curr Gastroenterol Rep, 2021. 23(11): p. 18.
- Keck, S., et al., Lack of Mucosal Cholinergic Innervation Is Associated With Increased Risk of Enterocolitis in Hirschsprung's Disease. Cell Mol Gastroenterol Hepatol, 2021. 12(2): p. 507-545.
- Moesch, M., et al., Associations of Mucosal Nerve Fiber Innervation Density with Hirschsprung-Associated Enterocolitis: A Retrospective Three-Center Cohort Study. Eur J Pediatr Surg, 2023. 33(4): p. 299-309.
- Volkart, S., et al., AQP1 in the Gastrointestinal Tract of Mice: Expression Pattern and Impact of AQP1 Knockout on Colonic Function. Int J Mol Sci, 2023. 24(4).
- Liao, S., et al., The regulatory roles of aquaporins in the digestive system. Genes & Diseases, 2021. 8(3): p. 250-258.