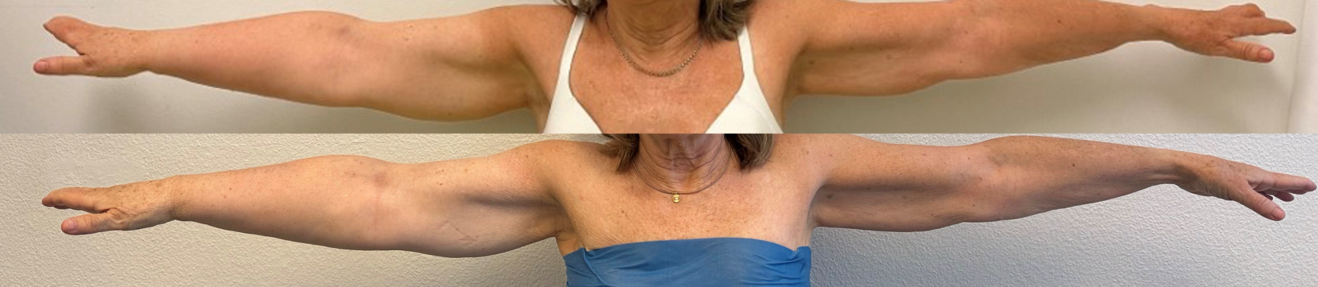
Lymphedema is a chronic, progressive condition characterized by painful swelling and reddening of an extremity due to disruption of the lymphatic system (Figure 1).1 Over time, it can result in inflammation, fibrosis, and recurrent infections (e.g. cellulitis, erysipelas). If left untreated, the disease may progress to irreversible structural damage, with significant functional and psychosocial consequences and reductions in long-term quality of life. Although conservative treatment remains a key component of symptom control, many patients continue to experience persistent complications.2-4 However, the recent emergence of reconstructive microsurgical techniques has redefined treatment goals, shifting the focus from palliative symptomatic management to physiological restoration of lymphatic drainage.5
Clinical presentation
The clinical presentation of lymphedema is highly variable in both symptoms and severity. Symptoms include limb swelling, a feeling of heaviness, recurrent infections (e.g. cellulitis or erysipelas), skin changes such as hyperkeratosis, pain, and also report a substantial psychological burden associated with reduced quality of life.6 In developed countries, secondary lymphedema is the most prevalent form, most commonly occurring after oncological treatments such as radical lymph node dissection or radiotherapy. In breast cancer in particular, de-escalation strategies in axillary lymph node dissections and radiotherapy have significantly reduced its incidence; however, depending on the exact treatment combination, between 10% and 30% of patients are still affected.7 Despite increasing evidence for the effectiveness of surgical interventions, especially when performed early, awareness of these treatment options remains limited among many health care providers. As a result, patients often remain untreated for far too long, although timely surgical management can considerably improve outcomes. Beyond symptom control, the goal is also to reduce the need for conservative therapy, which is labor-intensive, time-consuming. Given the complex nature of lymphedema, close collaboration within an interdisciplinary team is required to achieve optimal patient results.
Diagnostic workup
Diagnosis of lymphedema can be challenging, often delayed by clinical overlap with other causes of limb swelling such as chronic venous insufficiency or lipedema. Consequently, a thorough clinical history and physical examination are essential, particularly in high-risk oncological patients. Lymphedema is clinically classified based on clinical presentation according to the International Society of Lymphedema (ISL), which categorizes disease progression from subclinical changes to advanced disease.8 The ISL stages are as follows:
– Stage 0 (latent/subclinical stage): Lymphatic transport is impaired, but no visible swelling is present. Patients may report a sensation of heaviness or discomfort in the affected limb.
– Stage 1 (reversible stage): Onset of soft, pitting edema that is typically reversible with limb elevation or compression. Swelling may fluctuate throughout the day.
– Stage 2 (irreversible stage): Edema becomes more persistent and no longer resolves with elevation alone. Fibrotic changes begin to develop, and the tissue may feel firm or rubbery.
– Stage 3 (elephantiasis): The most advanced stage, characterized by severe swelling, extensive fibrosis, and irreversible skin changes such as hyperkeratosis, papillomatosis, and dermal thickening. Mobility may be significantly impaired, and recurrent infections are common.
Accurate quantification of limb volume is also essential for diagnosis and postoperative monitoring of lymphedema progression and/or response to therapy. Commonly used methods include:
(A) Limb circumference measurement: Circumference measurements are taken at specific anatomical landmarks (e.g., ankle, mid-calf, mid-thigh) using a measuring tape.5
(B) Water displacement volumetry: This method involves submerging the affected limb in a water tank and measuring the volume of displaced water.9 While time-intensive and impractical for the clinical setting, it provides a precise estimate of limb volume.
(C) 3D surface imaging technologies: Machine learning-based 3D scanners can generate a full three-dimensional model of the limb to calculate volume and detect contour irregularities, allowing for rapid, non-invasive, and highly reproducible assessments.10
Diagnostic imaging
Accurate diagnostics are essential not only for diagnosis and staging of lymphedema, but also essential for individualized surgical planning. Consequently, we commonly use a stepwise approach beginning with non-invasive, bedside tools and advancing to specialized imaging as needed.
(A) Ultrasound: Doppler ultrasound is essential to exclude venous pathologies such as deep vein thrombosis or chronic venous insufficiency that can mimic lymphedema. It can also detect minimal changes in soft tissue edema and ibrosis.11
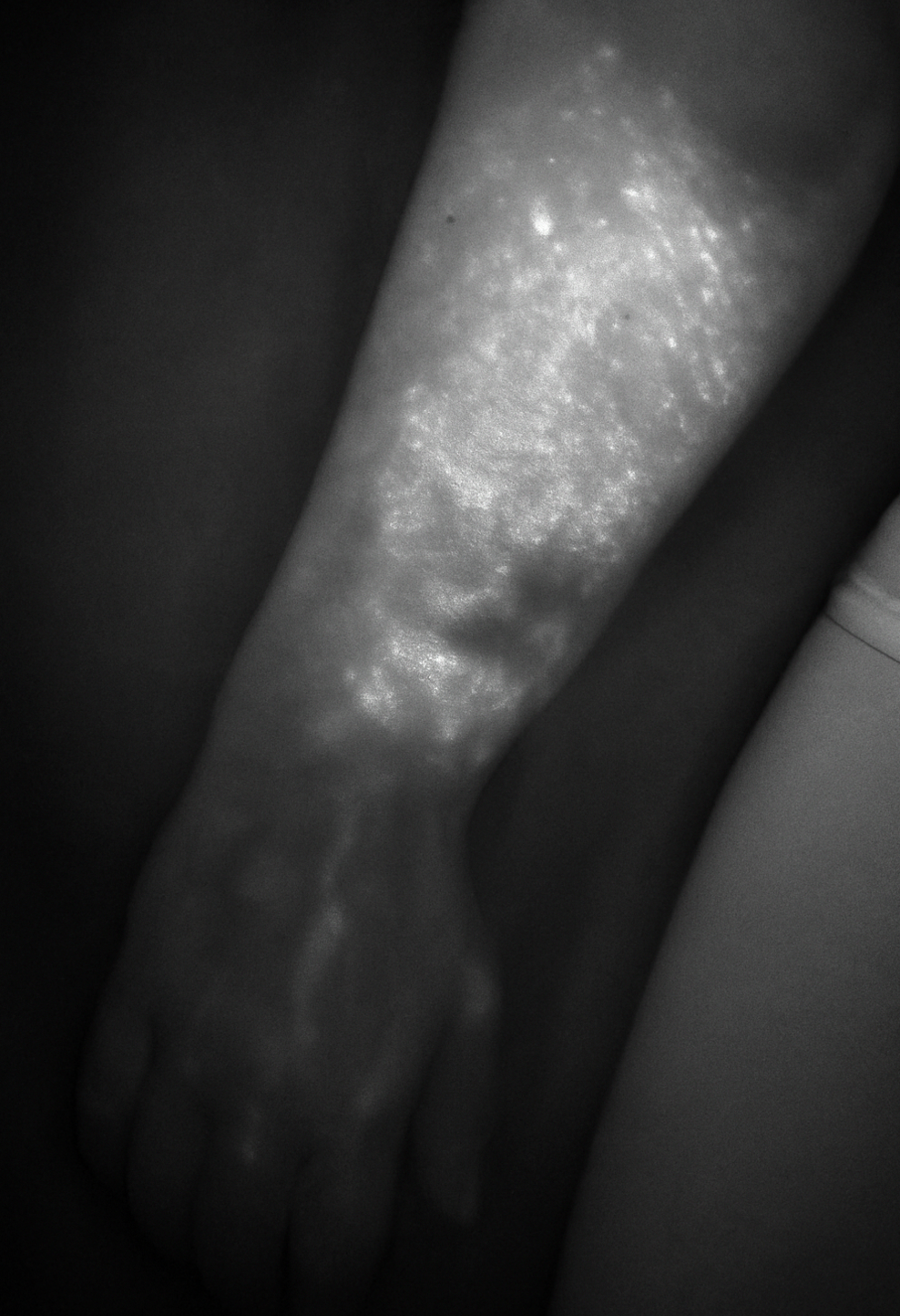
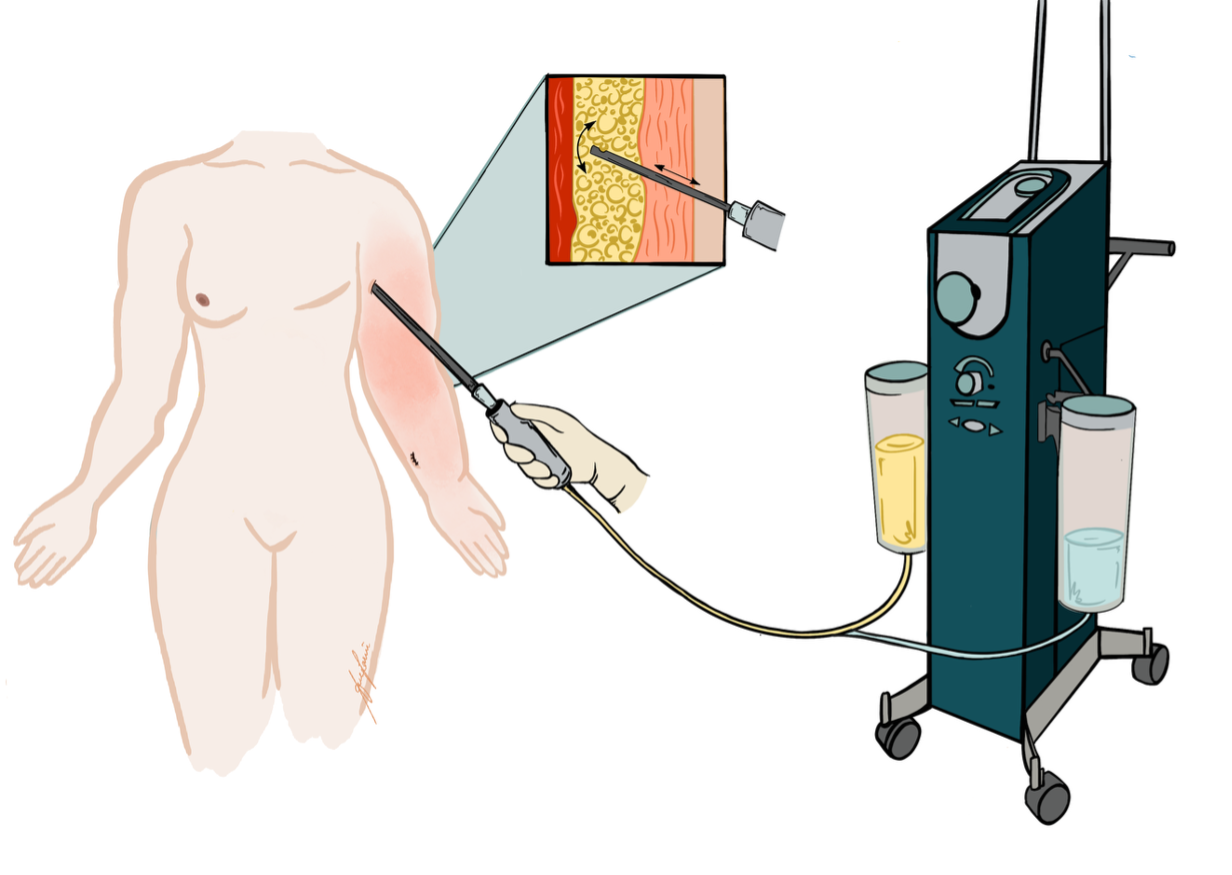
(B) Indocyanine Green (ICG) Lymphography: ICG lymphography is currently considered the gold standard for initial diagnostics. It is a real-time, minimally invasive imaging technique where near-infrared fluorescent dye is injected intradermally. Using specialized cameras, clinicians can visualize superficial lymphatic pathways, detect dermal backflow, and thus detect irregularities in lymph flow (Figure 2).12 It is particularly valuable for surgical planning (e.g., identifying potentially suitable lymphatic vessels for LVA).
(C) MRI and CT Lymphangiography: MRI and CT offer high-resolution anatomical imaging of both superficial and deep lymphatic vessels. MRI lymphangiography provides superior soft tissue contrast and can visualize lymph nodes, fluid accumulation, and fibrosis without radiation exposure.13 CT lymphangiography, although involving ionizing radiation, offers rapid acquisition and high spatial resolution, particularly useful in preoperative planning. Both modalities can be enhanced with intranodal contrast agents to directly image lymphatic channels but are less commonly used due to technical complexity and resource requirements.
(D) Lymphoscintigraphy: Lymphoscintigraphy involves subcutaneous injection of radiolabeled tracers (typically Tc-99m-labeled colloid), followed by gamma camera imaging to assess tracer uptake and drainage through lymphatic channels.14 It identifies functional abnormalities and can distinguish between primary and secondary lymphedema.
Treatment
Treatments can be categorized in conservative, reconstructive and ablative approaches. The three modalities can also be combined to achieve optimal results.15
Conservative Treatment
The gold standard of lymphedema treatment includes complex decongestive therapy (CDT), which includes:
– manual lymph drainage
– compression therapy and garments
– exercise and physiotherapy
– skin hygiene to prevent infection
CDT can reduce limb volume and improve symptoms but does not address the underlying pathophysiology and consequently requires lifelong adherence to regular follow-ups.3,4
Reconstructive Treatment
In contrast to CDT, (super-)microsurgical interventions aim to restore lymphatic drainage capacity by reconstructing lymphatic networks. These techniques have demonstrated long-term reductions in limb circumference, decreased risk of infection, lower number of CDT sessions per week and consequently improved quality of life.5 Commonly used surgical interventions include:
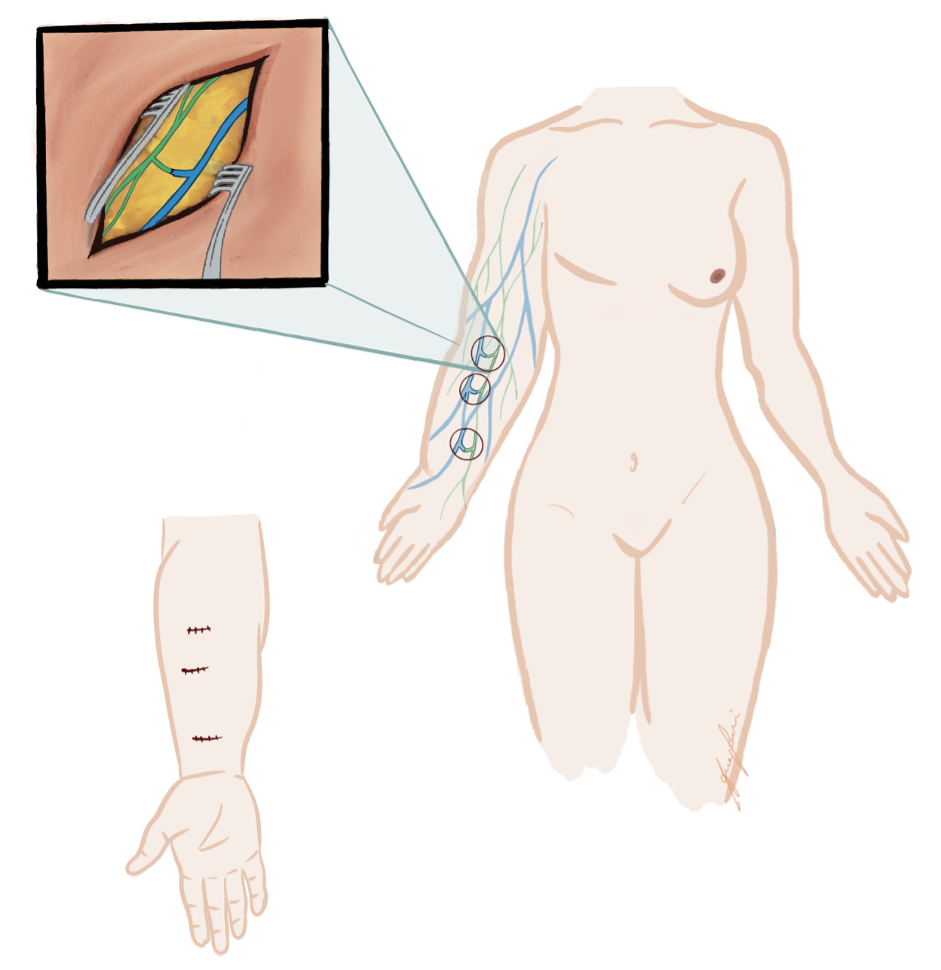
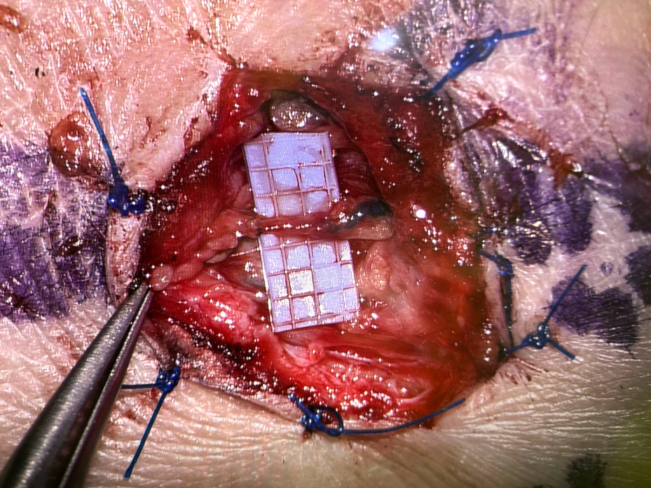
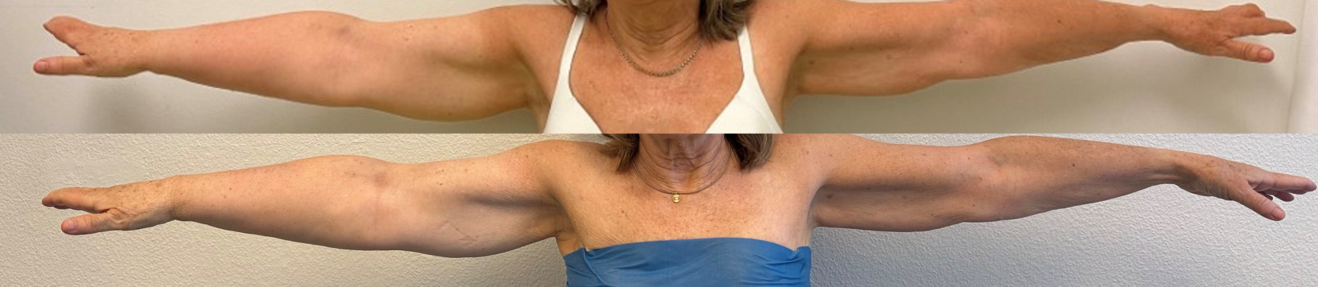
(A) Lymphaticovenous anastomosis (LVA): LVA is a supermicrosurgical procedure in which lymphatic vessels are connected to nearby subdermal venules, creating a distal shunt allowing for immediate lymphatic outflow (Figure 3).16,17 It is most effective in early-stage lymphedema (ISL stages I–II), before irreversible fibrotic changes of lymphatic vessels occur. LVA is minimally invasive, may also be performed under local anesthesia, and often results in rapid symptom relief and volume reduction with low complication rates (Figure 4). Real-time indocyanine green (ICG) lymphography is typically used to guide vessel selection.
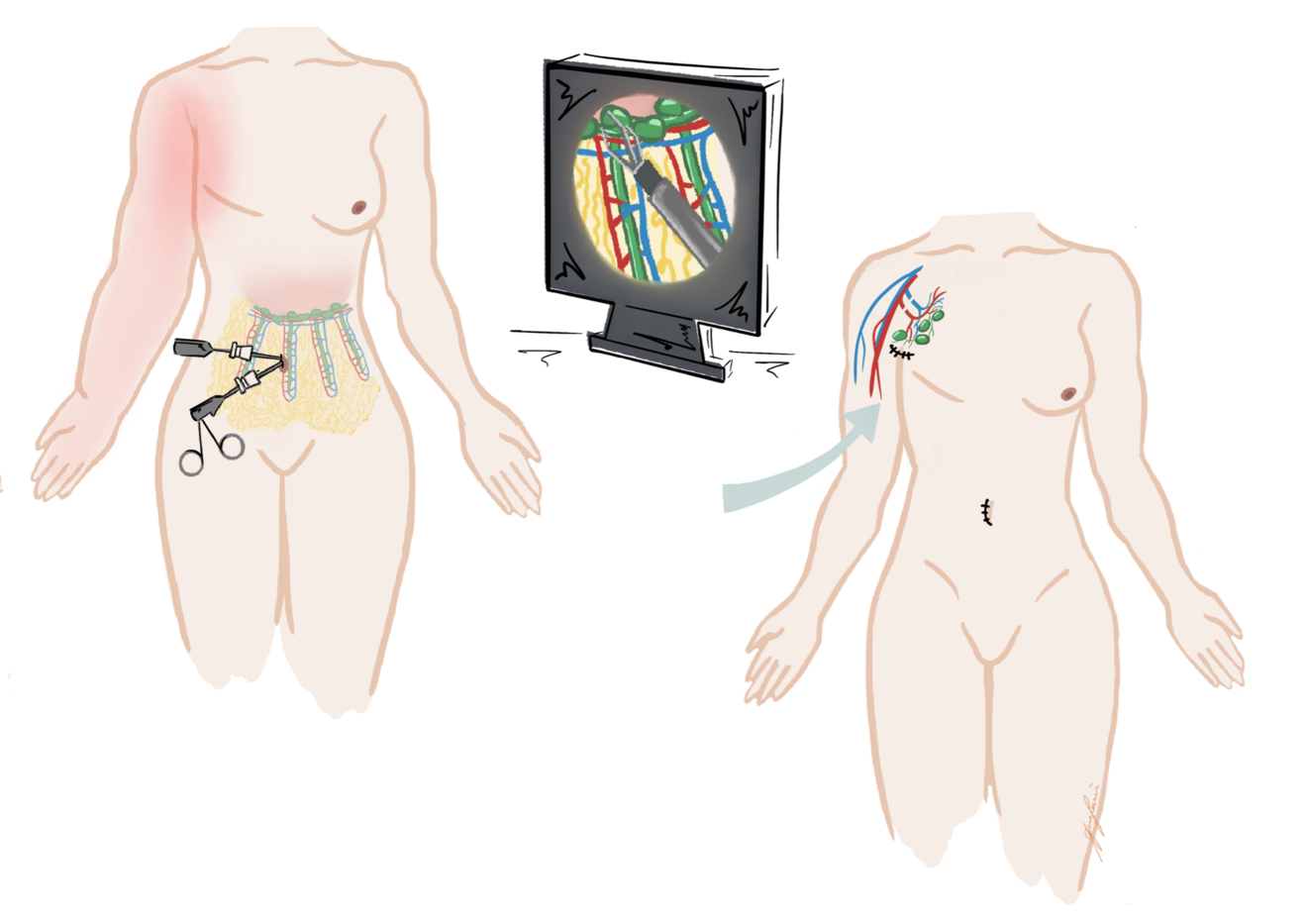
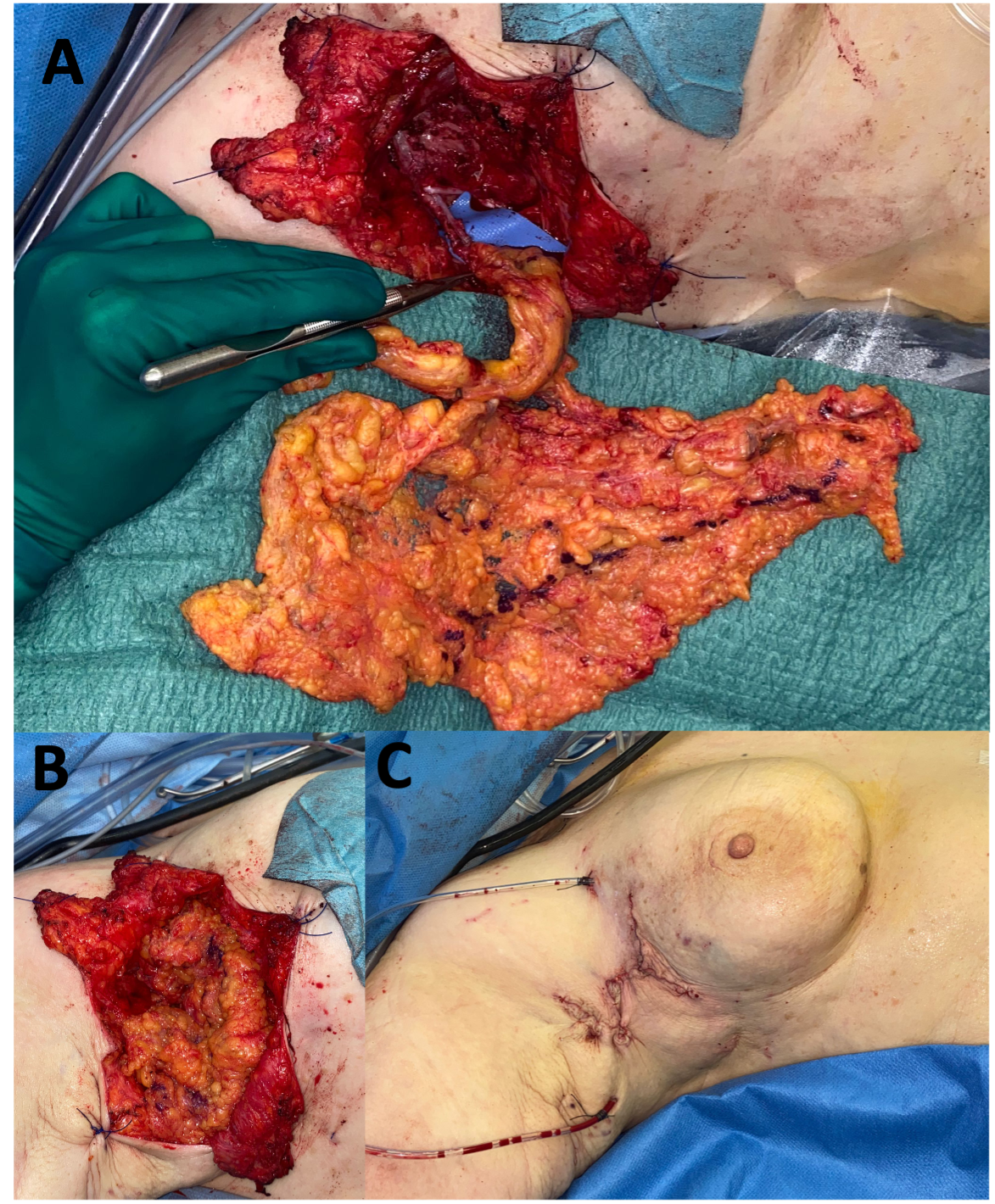
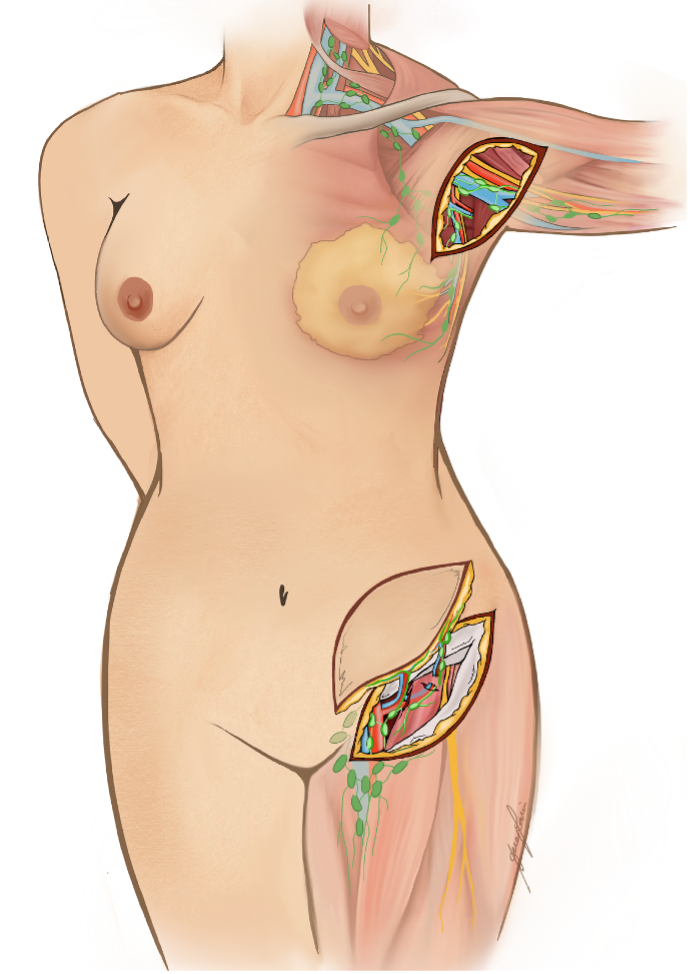
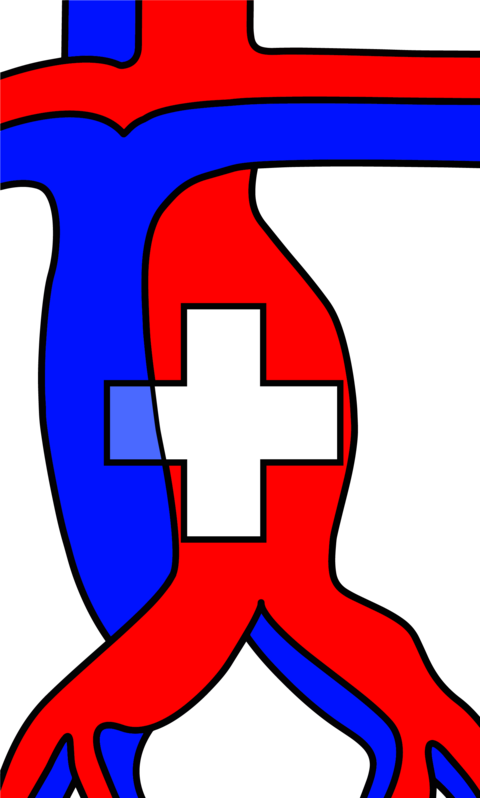
(B) Vascularized lymph node transfer (VLNT): VLNT involves harvesting lymph nodes from a healthy donor site (e.g., groin, neck, or omentum) with their vascular supply and transplanting them to the lymphedematous region (Figure 5).18 The transferred nodes are believed to secrete vascularized endothelial growth factors (e.g. VEGF-C), which promote local lymphangiogenesis, restoring drainage in areas with complete lymphatic disruption (Figure 6).19 This approach is also suited for more advanced disease (ISL stages II–III), particularly in patients with minimal or absent native lymphatic channels.20 Careful donor site selection after reverse lymphatic mapping is essential to minimize the risk of inducing iatrogenic lymphedema.
(C) Lymph Interpositional Flap Transfer (LIFT) / Vascularized Lymph Vessel Transfer (VLVT): In cases where large dead spaces require coverage with lymphatic tissue, the conventional microsurgical VLNT approach may not be optimal. As an alternative, free tissue transfer using lymphatic-rich flaps has recently been proposed for lymphatic reconstruction (Figure 7). Among these, the superficial circumflex iliac artery perforator (SCIP) flap has gained particular attention because it contains unidirectional lymphatic vessels oriented toward the inguinal lymph nodes.21-23 By transplanting functional lymphatic vessels, this technique not only restores lymphatic drainage but also provides reliable coverage of extensive tissue defects.
Ablative Treatment
Ablative methods (e.g. liposuction) is particularly helpful in combination with conservative and/or reconstructive approaches.
(A) Liposuction: Patients with advanced-stage lymphedema commonly suffer from excess fibrous tissue that cannot be resolved by reconstructive surgery alone. Liposuction serves as a valuable adjunct by mechanically debulking the excess tissue, resulting in immediate volume reduction.15 While liposuction does not improve lymphatic function per se, it can significantly enhance limb contour and patient comfort (Figure 8). Consequently, liposuction is commonly used as an adjunct to reconstructive procedures (Figure 9). Additionally, to sustain long-term results, patients must adhere to compression garments after procedure.
Current Research Strategies
Lymphedema is a rapidly advancing field with new discoveries being made on a weekly basis. Our LYMPH Trial www.lymphtrial.com is a worldwide multicentric, randomized controlled trial aimed at demonstrating that microsurgical treatment in combination with conservative therapy genuinely improves the quality of life and reduces lymphedema in patients with chronic lymphedema following breast cancer treatment, as current evidence is mostly limited to small retrospective cohort studies. Notably, the project was developed together with affected patients, thus prioritizing patient-reported outcomes. Additionally, in our multicentric lymphedema database we have been aiming to quantify improvements after surgery in hopes of identifying modifiable variables (e.g. ideal donor and recipient site selection, lymphatic vessel quality, etc.). Finally, our group directly works with affected patients to identify important research questions. With our studies, we aim to make a positive impact in both clinical measurements and also patient-reported quality of life by addressing the significant clinical challenges faced by cancer survivors every day.
Neurolymphatics – a future steppingstone
The lymphatic and nervous systems are increasingly recognized as interconnected regulators of tissue homeostasis, inflammation, and repair. Recent studies suggest that lymphatic vessels are not merely passive drainage conduits but are functionally linked to neural signaling pathways.24,25 In particular, meningeal lymphatic vessels have been implicated in clearing metabolic waste products from the central nervous system, thereby influencing neuroinflammation and neurovascular health.26,27 Emerging preclinical evidence indicates that enhancing lymphatic function may prevent cognitive decline and improve neurological outcomes in patients with Alzheimer’s disease and other neurodegenerative disorders.28,29 These findings highlight the potential of targeting the lymphaticoneural axis as a novel therapeutic strategy for maintaining brain health and delaying disease progression.
Conclusion
With lymphedema affecting every fifth breast and pelvic cancer survivors, reconstructive microsurgery has become an essential component of lymphedema management, offering restoration of lymphatic drainage capacity and significant improvements in patient outcomes (Figure 10). Conservative therapy alone is often insufficient; therefore, surgical options should be systematically considered and offered whenever appropriate. Consequently, we aim to integrate surgical solutions into standard lymphedema care pathways. To establish evidence-based best practices, multicenter randomized trials and collaborative databases, such as our LYMPH study and the international lymphedema registry, will be indispensable. Additionally, the rapidly advancing basic and translational research hold promise for preventive and curative treatments in the future.
Acknowledgements: We thank Ana Lariu for the illustrations of lymphatic reconstructive techniques.
- Grada AA, Phillips TJ. Lymphedema: Pathophysiology and clinical manifestations. J Am Acad Dermatol. Dec 2017;77(6):1009-1020. doi:10.1016/j.jaad.2017.03.022
- Vassard D, Olsen MH, Zinckernagel L, Vibe-Petersen J, Dalton SO, Johansen C. Psychological consequences of lymphoedema associated with breast cancer: a prospective cohort study. Eur J Cancer. Dec 2010;46(18):3211-8. doi:10.1016/j.ejca.2010.07.041
- Jeffs E, Ream E, Taylor C, Bick D. Clinical effectiveness of decongestive treatments on excess arm volume and patient-centered outcomes in women with early breast cancer-related arm lymphedema: a systematic review. JBI Database System Rev Implement Rep. Feb 2018;16(2):453-506. doi:10.11124/JBISRIR-2016-003185
- Heinig B, Wollina U. [Complex decongestive therapy]. Hautarzt. Nov 2015;66(11):810-8. doi:10.1007/s00105-015-3674-1
- Kappos EA, Fabi A, Halbeisen FS, et al. Vascularized lymph node transfer (VLNT) versus lymphaticovenous anastomosis (LVA) for chronic breast cancer-related lymphedema (BCRL): a retrospective cohort study of effectiveness over time. Breast Cancer Res Treat. Dec 10 2024;doi:10.1007/s10549-024-07567-5
- Fu MR, Ridner SH, Hu SH, Stewart BR, Cormier JN, Armer JM. Psychosocial impact of lymphedema: a systematic review of literature from 2004 to 2011. Psychooncology. Jul 2013;22(7):1466-84. doi:10.1002/pon.3201
- DiSipio T, Rye S, Newman B, Hayes S. Incidence of unilateral arm lymphoedema after breast cancer: a systematic review and meta-analysis. Lancet Oncol. May 2013;14(6):500-15. doi:10.1016/S1470-2045(13)70076-7
- Lymphology ECotISo. The diagnosis and treatment of peripheral lymphedema: 2020 Consensus Document of the International Society of Lymphology. Lymphology. 2020;53(1):3-19.
- Farina G, Galli M, Borsari L, Aliverti A, Paraskevopoulos IT, LoMauro A. Limb Volume Measurements: A Comparison of Circumferential Techniques and Optoelectronic Systems against Water Displacement. Bioengineering (Basel). Apr 15 2024;11(4)doi:10.3390/bioengineering11040382
- Mastick J, Smoot BJ, Paul SM, et al. Assessment of Arm Volume Using a Tape Measure Versus a 3D Optical Scanner in Survivors with Breast Cancer-Related Lymphedema. Lymphat Res Biol. Feb 2022;20(1):39-47. doi:10.1089/lrb.2020.0119
- Pirri C, Pirri N, Ferraretto C, et al. Ultrasonographic Anatomy and Examination of the Subcutaneous Tissue (in Lymphedema). J Ultrasound Med. Jun 12 2025;doi:10.1002/jum.16742
- Narushima M, Yamamoto T, Ogata F, Yoshimatsu H, Mihara M, Koshima I. Indocyanine Green Lymphography Findings in Limb Lymphedema. J Reconstr Microsurg. Jan 2016;32(1):72-9. doi:10.1055/s-0035-1564608
- Kageyama T, Miyazaki T, Sakai H, Tsukuura R, Yamamoto T. Noncontrast magnetic resonance imaging-based evaluation of quality of life in secondary upper extremity lymphedema. J Vasc Surg Venous Lymphat Disord. Mar 04 2025;13(4):102220. doi:10.1016/j.jvsv.2025.102220
- Fei Y, Lu Y, Yao Z, Yin K, Zhou D, Liu Z. Diagnostic Value of Multimodal Lymphatic Imaging Techniques in Thoracic Duct Outlet Obstruction. Diagnostics (Basel). May 20 2025;15(10)doi:10.3390/diagnostics15101288
- Ghazaleh AA, Handschin TM, Buckowiecki J, et al. Combining reconstructive and ablative surgical treatment of chronic breast cancer-related lymphedema (BCRL): safe and effective. Breast Cancer Res Treat. Jan 2023;197(1):83-92. doi:10.1007/s10549-022-06778-y
- Qiu SS, Pruimboom T, Cornelissen AJM, Schols RM, van Kuijk SMJ, van der Hulst RRWJ. Outcomes following lymphaticovenous anastomosis (LVA) for 100 cases of lymphedema: results over 24-months follow-up. Breast Cancer Res Treat. Nov 2020;184(1):173-183. doi:10.1007/s10549-020-05839-4
- Scaglioni MF, Arvanitakis M, Chen YC, Giovanoli P, Chia-Shen Yang J, Chang EI. Comprehensive review of vascularized lymph node transfers for lymphedema: Outcomes and complications. Microsurgery. Feb 2018;38(2):222-229. doi:10.1002/micr.30079
- Schaverien MV, Badash I, Patel KM, Selber JC, Cheng MH. Vascularized Lymph Node Transfer for Lymphedema. Semin Plast Surg. Feb 2018;32(1):28-35. doi:10.1055/s-0038-1632401
- Subramanyam P, Janarthanan R, Palaniswamy SS. Early Demonstration of Spontaneous Perinodal Lymphangiogenesis by Lymphoscintigraphy after Vascularized Lymph Node Transplantation - A Pilot Study. Indian J Nucl Med. 2022;37(1):1-6. doi:10.4103/ijnm.ijnm_123_21
- Seidenstuecker K, Fertsch S, Ghazaleh AA, et al. Improving quality of life after breast cancer: a comparison of two microsurgical treatment options for breast cancer-related lymphedema (BCRL). Clin Exp Med. Apr 23 2024;24(1):82. doi:10.1007/s10238-024-01344-w
- Campos JL, Suominen S, Pons G, et al. Lymphatic Patterns in the Superficial Circumflex Iliac Artery Perforator Flap. J Reconstr Microsurg. Mar 2025;41(3):209-218. doi:10.1055/a-2340-9629
- Koshima I, Nanba Y, Tsutsui T, et al. Superficial circumflex iliac artery perforator flap for reconstruction of limb defects. Plast Reconstr Surg. Jan 2004;113(1):233-40. doi:10.1097/01.PRS.0000095948.03605.20
- Yoshimatsu H, Cho MJ, Karakawa R, et al. The role of lymphatic system transfer (LYST) for treatment of lymphedema: A long-term outcome study of SCIP flap incorporating the lymph nodes and the afferent lymphatic vessels. J Plast Reconstr Aesthet Surg. Feb 2025;101:15-22. doi:10.1016/j.bjps.2024.11.052
- Kappos EA, Haas Y, Schulz A, et al. The LYMPH trial: comparing microsurgical with conservative treatment for chronic breast cancer-associated lymphoedema - study protocol of a pragmatic randomised international multicentre superiority trial. BMJ Open. Feb 17 2025;15(2):e090662. doi:10.1136/bmjopen-2024-090662
- González-Hernández S, Mukouyama YS. Lymphatic vasculature in the central nervous system. Front Cell Dev Biol. 2023;11:1150775. doi:10.3389/fcell.2023.1150775
- Absinta M, Ha SK, Nair G, et al. Human and nonhuman primate meninges harbor lymphatic vessels that can be visualized noninvasively by MRI. Elife. Oct 03 2017;6doi:10.7554/eLife.29738
- Ahn JH, Cho H, Kim JH, et al. Meningeal lymphatic vessels at the skull base drain cerebrospinal fluid. Nature. Aug 2019;572(7767):62-66. doi:10.1038/s41586-019-1419-5
- Da Mesquita S, Louveau A, Vaccari A, et al. Functional aspects of meningeal lymphatics in ageing and Alzheimer's disease. Nature. Aug 2018;560(7717):185-191. doi:10.1038/s41586-018-0368-8
- Louveau A, Plog BA, Antila S, Alitalo K, Nedergaard M, Kipnis J. Understanding the functions and relationships of the glymphatic system and meningeal lymphatics. J Clin Invest. Sep 01 2017;127(9):3210-3219. doi:10.1172/JCI90603
- Wang L, Zhang Y, Zhao Y, Marshall C, Wu T, Xiao M. Deep cervical lymph node ligation aggravates AD-like pathology of APP/PS1 mice. Brain Pathol. Mar 2019;29(2):176-192. doi:10.1111/bpa.12656