Case description
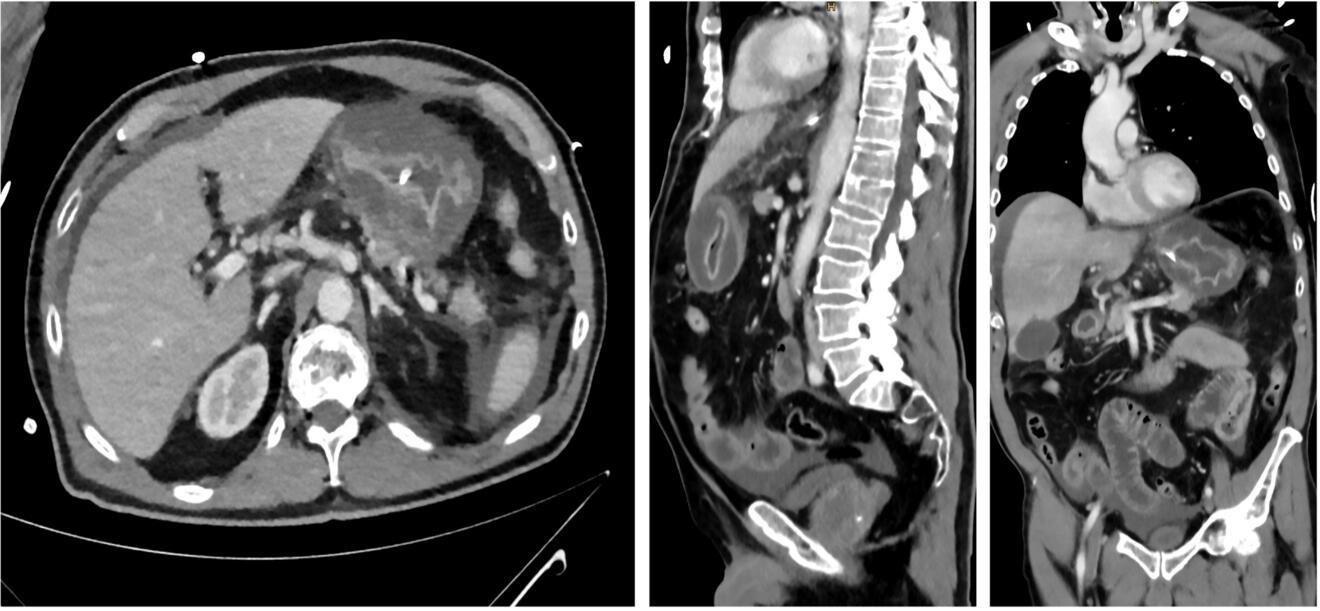
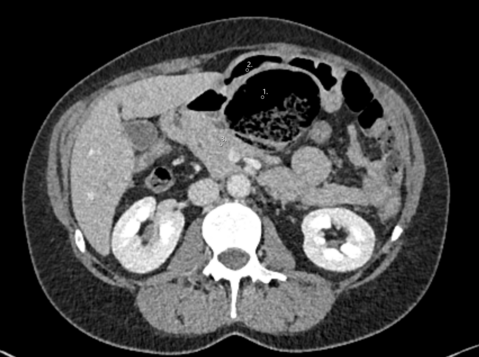
A 76-year-old patient was referred to our emergency department by a local gastroenterologist because of worsening abdominal pain and elevated inflammatory parameters. The patient had undergone an upper endoscopy with biopsies two days previously where an infection with Helicobacter pylori was diagnosed. The patient reported about gradually worsening epigastric abdominal pain over the last two days, as well as nausea and vomiting. He had no relevant comorbidities and did not take any regular medication. On admission the patient was hemodynamically unstable with a blood pressure of 80/67mmHg and tachycardia of 113/min but afebrile. The physical exam revealed strongly diminished bowel sounds as well as a distended, tense and tender abdomen. Furthermore, mottling around both knees was present. The cardiac, pulmonary as well as neurological clinical exam were unremarkable. Initial laboratory values showed a white blood cell count of 1.4 G/L, a c-reactive protein level of 448 mg/L and lactate of 7.9 mmol/L. Aspartate transaminase was 34 U/L, alanine transaminase was 12 U/L and the total bilirubin was 37 µmol/L. Additionally creatinine was elevated at 276 µmol/L. Haemoglobin and electrolytes were normal. A point of care ultrasound revealed free peritoneal fluid. An empiric antibiotic therapy with piperacillin-tazobactam as well as fluid resuscitation was initiated immediately. A contrast enhanced computed tomography of the chest and abdomen was performed which showed free fluid in the abdomen and a diffuse thickening of the stomach wall (Fig. A).
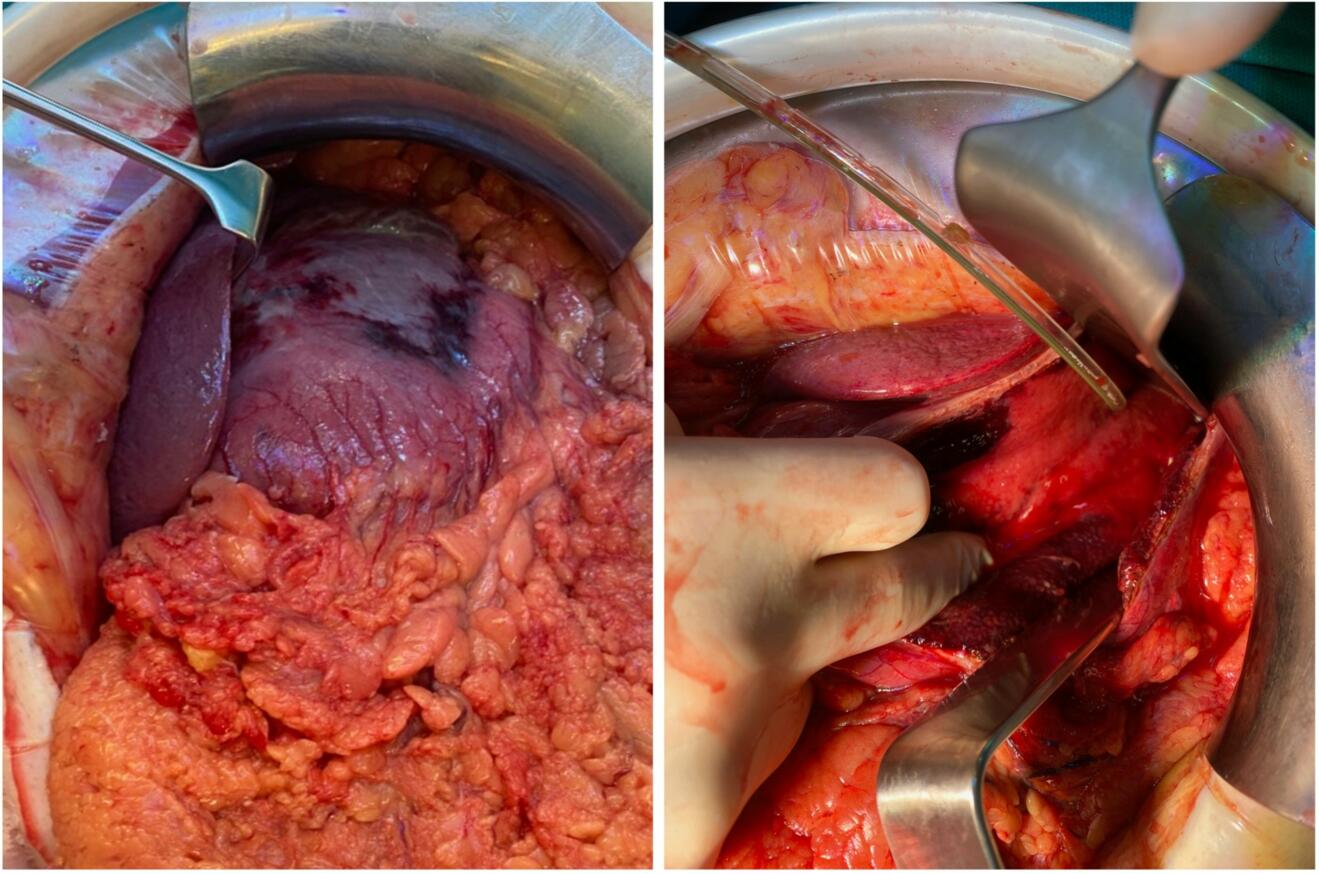
The patient was transferred to the intensive care unit for further management. During the first 24 hours the patient showed a massive volume requirement and increasing need for vasopressors. Due to progressive respiratory distress he was intubated and an increasing intraabdominal pressure of up to 30 mmHg was noted. At this point the indication for a laparotomy with open abdomen treatment was established. Intraoperatively, part of the stomach wall presented necrotic. Due to vital appearing mucosa in the rest of the stomach a necrosectomy was performed instead of a gastrectomy (Fig. B). Cultures were obtained intraoperatively and growth of Streptococcus pyogenes was found.
What is the most likely diagnosis?
- Gastric carcinoma
- Phlegmonous gastritis
- Gastric perforation after endoscopic biopsy
- MALT lymphoma
- Gastric ulcer
Case solution
The patient suffered from acute phlegmonous gastritis with streptococcal toxic-shock syndrome complicated by an abdominal compartment syndrome and severe acute kidney injury.
Phlegmonous gastritis is a rare bacterial infection of the gastric wall, around 500 cases have been previously published (1) and since 1980 only 167 results can be found on PubMed. The main pathogens are streptococcus species (e.g. Streptococcus pyogenes) which have been isolated in about 70% of cases and are associated with lethal outcomes (1). However, other pathogens such as staphylococcus species, enterococcus species, moraxella species or polymicrobic infections have also been reported (2, 3, 4). The aetiology is not fully understood but possible infection routes include local invasion into the stomach or haematogenous spread. Mucosal injuries are known risk factors for phlegmonous gastritis (5), these can be present during chronic gastritis, gastric ulcers, gastric malignancy or after endoscopic biopsies. Additional risk factors include immunosuppression, chronic alcohol abuse and diabetes mellitus (6). Mortality rates of up to 54% have been reported (1), some authors differentiate between localized and diffuse phlegmonous gastritis where a localized infection has a notably lower mortality (10% vs. 54%) (2). Diagnosis can be made by computed tomography, endoscopy or intraoperatively. The key to successful management is early recognition and treatment since progression of the disease can be very rapid. Because of the limited experience with this disease no treatment guidelines exist. Generally, fluid therapy and broad-spectrum antibiotics are the recommended initial treatment (7, 8). In the presence of complications (e.g. perforation) or progression of disease despite conservative treatment, surgery should be performed immediately, approximately 40% of patients need surgical intervention (1). If possible, it is advised to perform local resections, however total gastrectomy is often performed due to diffuse infection (1, 5, 9).
In our case two more surgeries with further necrosectomy were necessary. The open abdomen treatment was concluded after 14 days. We were able to start enteral feeding and discharge the patient from the intensive care unit after 88 days. Unfortunately, renal function did not return and the patient currently undergoes haemodialysis as a kidney replacement therapy.
Reviewed by
Christian Nebiker, Editorial Board and Matthias Pfister, Editorial Team
1. Rada-Palomino A, Munoz-Duyos A, Perez-Romero N, Vargas-Pierola H, Puertolas-Rico N, Ruiz-Campos L, et al. Phlegmonous gastritis: A rare entity as a differential diagnostic of an acute abdomen. Description of a case and a bibliographic review. Rev Esp Enferm Dig. 2014;106(6):418-24.
2. Yang H, Yan Z, Chen J, Xie H, Wang H, Wang Q. Diagnosis and treatment of acute phlegmonous gastritis: A case report. Medicine (Baltimore). 2018;97(18):e0629.
3. Kakimoto S, Harada Y, Shimizu T. Phlegmonous gastritis. CMAJ. 2023;195(35):E1181.
4. Iwata N, Adachi Y, Yoshida Y, Ishii Y, Endo T. Phlegmonous Gastritis in a Patient With Nonalcoholic Steatohepatitis-Related Cirrhosis: A Case Report and Review of Literature. Cureus. 2023;15(1):e33551.
5. Paramythiotou E, Mitrakou C, Savva A, Antoniadou A, Armaganidis A, Dimopoulos G. Phlegmonous Gastritis and Streptoccocal Toxic Shock Syndrome: An Almost Lethal Combination. Indian J Crit Care Med. 2021(10):1197-200.
6. Ramphal W, Mus M, Nuytinck HKS, van Heerde MJ, Verduin CM, Gobardhan PD. Sepsis caused by acute phlegmonous gastritis based on a group A Streptococcus. J Surg Case Rep. 2018;2018(8):rjy188.
7. Czapka MT, Schrantz SJ. Phlegmonous gastritis: Evolving from surgical to medical disease. IDCases. 2023;32:e01777.
8. Durdella H, Everett S, Rose JA. Acute phlegmonous gastritis: A case report. J Am Coll Emerg Physicians Open. 2022;3(2):e12640.
9. Kobus C, Van den Broek J, Richir M. Acute gastric necrosis caused by a beta-hemolytic streptococcus infection: a case report and review of the literature. Acta Chir Belg. 2020.