Quality measurement in vascular surgery is crucial for enhancing patient outcomes, optimizing healthcare resource utilization, and ensuring the safety and effectiveness of invasive vascular interventions (1). As these procedures become increasingly complex, the demand for robust quality metrics rises accordingly. Such metrics are essential for objectively evaluating performance, guiding clinical decision-making, and shaping healthcare policies. Additionally, adherence to clinical practice guidelines—such as intervention thresholds and procedural volume standards—should be systematically monitored to maintain high standards of care. Establishing reliable benchmarks is essential for experts to systematically compare their clinic’s performance with that of other institutions. Therefore, the development of reliable quality metrics has become a priority to provide benchmarks for surgical practice, foster accountability among healthcare providers, and ultimately enhance patient care.
Challenges remain in measuring outcome parameters, such as complications (e.g. cardiac or neurovascular), long-term outcome and survival, re-intervention rate, functional recovery, and amputation free survival. However, how can we provide the physicians and clinics with the quality metrics that are important to them? What data do we need for this? And what is required to make these analyses reliable?
This paper provides an overview of the existing quality control measures in vascular surgery across Switzerland, as coordinated by the Swiss Society of Vascular Surgery (SSVS). It discusses the benefits and challenges encountered in practice and showcases a pilot project in the Canton of Zurich, which serves as a model for potential nationwide expansion.
What data is currently used?
The primary data source for quality control in vascular surgery is the dedicated clinical registry (Swissvasc) of the SSVS, which is filled by most of the clinics in Switzerland. This registry is presented in the next section. In a pilot project and parallel to the registry, administrative data are also used in this context, although this data has not specifically been collected for the purpose of quality assurance. Administrative data and the advantage of linking the two data sources are presented in a subsequent section.
Swissvasc - The Swiss Vascular Registry
Along with international trends of establishing registries for quality control (2) the SSVS established a registry in 2003 called Swissvasc (3). In 2015 the IT provider was changed, resulting in a fully web-based system called Swissvasc 2.0, hosted by "Adjumed Services AG". Swissvasc 2.0 is designed in a matrix structure, allowing any vascular procedure - open or endovascular - arterial or venous - standard or patient-specific- primary or secondary intervention - to be recorded. The registry has been designed and further developed in accordance with the international VASCUNET templates, regarding the type and granularity of parameters to be collected in a vascular registry(4). The participation in the registry is voluntary, except for hospitals offering training in vascular surgery and for hospitals with a service mandate in the Cantons of Zurich and St. Gallen, where specific quality programs for abdominal aortic aneurysms (AAA) and carotid interventions are established.
The registry demonstrates an almost complete case coverage. A recently conducted VASCUNET audit in five hospitals across four Cantons (covering around 50% of all cases operated in Switzerland) confirmed that more than 99% of aortic, carotid and peripheral bypass procedures were captured. Besides the excellent case ascertainment, the quality of the entered data was reasonably good with more than 90% of the collected parameters being correctly recorded when compared to clinical records in these hospitals (5,6).
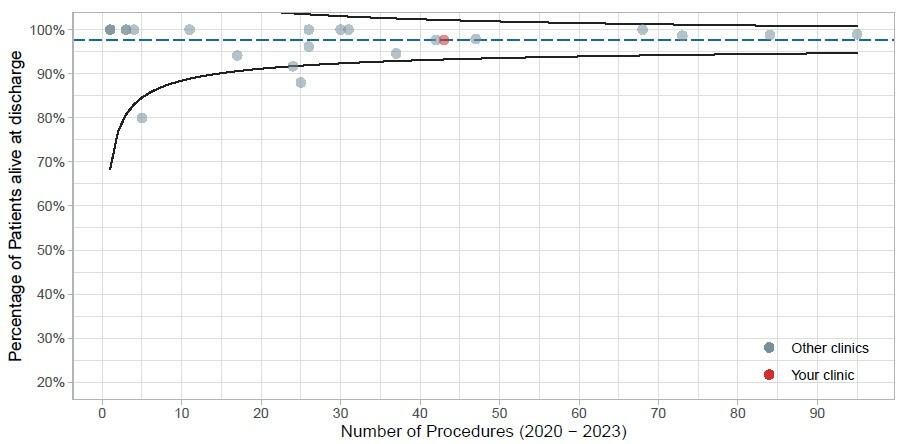
To describe the quality, a clinic-specific annual report and a national annual report are published, providing data on volume, risk factors, quality of indication and in-hospital outcomes at discharge (7). As an example, Figure 1 illustrates a funnel plot of hospital volume and survival at discharge for elective infrarenal aortic aneurysms repairs.
The Value of the Registry
The registry allows benchmarking of treatment indications, outcomes and hospital volume among participating clinics and provides a comparison over time. Thereby, the mentioned parameters can be analyzed for any specific clinic or for Switzerland in general. Thanks to the cooperation with VASCUNET, benchmarking as a country could be carried out in several studies (8–11). In that way, Swissvasc helps identifying trends in the treatment provided and allows identification of changes in treatment-specific outcomes over a period of time in a local, national, and even international context.
Benefits of the Registry
– Benchmarking of hospitals at the level of volume, outcome, diagnostic quality
– Practice patterns over time
– International benchmarking
– Research and publications
– Annual reports
– Logbook for surgeons
Besides that, Swissvasc serves as a valuable data source for high-quality epidemiological and clinical research in vascular surgery. For example, an epidemiological analysis revealed that thoracic aortic surgery is already highly centralized in Switzerland, whereas procedures on the abdominal aorta are performed in approximately 30 clinics, with 50% not meeting the recommended annual caseload (12,13). A recently published study identified associations between various organ specific complications and morbidity or mortality following complex abdominal aortic surgery in Switzerland (14). After the recent VASCUNET audit that confirmed the validity of the captured data, Swissvasc is ready to serve as a valuable data source for high-quality research with international impact.
Lastly, Swissvasc allows a person-specific export of all performed procedures in a specific period into a personal logbook (www.swissvasc-logbook.ch). The logbook export is an automatically generated look-alike to the official Swiss Institute for Medical Education and Training (SIWF) logbook and therefore serves as a practical tool for surgeons in training to capture and track their progress.
Challenges and Limitations of the Registry
Entering data into the registry is time consuming – particularly when it comes to risk factors of patients. To expedite patient registration, several tools have been implemented. First, an interface between the patient record system and the registry allows adding new cases in the registry along with basic patient information (age, year of birth, patient and case identification numbers) by one click. Furthermore, the use of standardized templates allows for the rapid documentation of most procedures, requiring only minimal manual input, such as details on indication or anatomical measurements (e.g., aneurysm diameter). These tools facilitate the efficient recording of even complex, multi-step procedures.
Challenges of the Registry
– Time consuming
– Follow up data and long-term data can be missing
– No linkage if patients change hospital during process
The data quality is reasonably good for parameters captured up until discharge, including in-hospital complications. However, the effort to capture clinical follow up data and information on long-term outcomes is currently very high. The problem is even more pronounced when complications or consecutive interventions for the same patient and pathology are performed in different hospitals. In that circumstance, the patient will be captured with two different hospital patient identifiers and a linkage is currently not possible. Therefore, not all data are available for valid long-term outcomes, such as reintervention rates, (amputation-free) survival or patency rates of vascular reconstructions.
Since participation in the registry is voluntary in most regions, the risk of incomplete data collection remains significant. To support the SSVS in addressing this challenge and to facilitate reliable quality analyses, the health authorities of the Canton of Zurich have launched a quality monitoring and improvement initiative. This initiative aims to ensure comprehensive monitoring of vascular interventions and to integrate clinical parameters with administrative health data.
Administrative Health Data
In addition to manually collected registry data, healthcare systems also generate extensive administrative health data. Although these data are not primarily collected for quality control purposes, they provide a comprehensive source of all interventions with detailed information, including the type and exact timing of procedures (surgical procedures codes with timestamps), corresponding diagnoses (ICD-10), complications, patient demographics, financial expenditures and revenues, as well as information about the operating surgeons.
Moreover, treatments received at different hospitals can be linked to individual patients, enabling a longitudinal analysis of their healthcare journey. Additionally, complications that arise during hospitalization can be distinguished from pre-existing comorbidities present at admission. One particularly valuable aspect of these data is the ability to determine whether a patient has died after hospital discharge. Part of that data has extensively been used for epidemiological research in vascular surgery in Switzerland.(15–18)
The primary advantage of administrative health data is that they are routinely collected and can be utilized without additional effort. However, a major limitation is that their availability varies across different cantons. Furthermore, information on out-of-hospital mortality of former patients is currently not accessible in Switzerland due to data protection regulations. At present, cantonal health authorities can only reliably track in-hospital mortality, including the cause of death.
A significant limitation is that the SSVS does not have direct access to these raw data. Data protection regulations in Switzerland are highly rigid, necessitating alternative approaches to data utilization. However, within the framework of a joint quality program with the Canton of Zurich, it is possible to link both datasets, enabling comprehensive quality assessments.
Other limitations include that the used ICD-10 codes do not necessarily represent clinically established classifications. For instance, the widely accepted Stanford classification for aortic dissections is not reflected; instead, ICD-10 codes differentiate between thoracic, thoracoabdominal, and abdominal aortic dissections. More importantly, only data on hospitalizations are captured, while data on outpatient treatments (outside of hospital) are exclusively sent to patients’ health insurance company but not to health authorities. However, this limitation may become obsolete starting January 2028, when the financing of inpatient and outpatient services is set to be standardized, allowing health authorities to likely capture all billed medical procedures (19).
Probably the main limitation, however, is the lack of specific, essential clinical parameters in the administrative data. Examples for lacking clinical parameters are the diameter of AAA's, the presence of endoleaks, which are essential when evaluating indication quality and comparing treatment outcomes. The lack of these clinical parameters complicates the identification of patients with specific clinical conditions such as "symptomatic carotid stenosis" or endoleaks following endovascular aortic repair (EVAR). Complex combination of ICD and CHOP codes might be needed to identify such patient groups to assure fair comparisons. In contrast – Swissvasc specifically captures most clinically relevant information and thereby allows identification and monitoring of patients with these clinical conditions.
Why do we need both Registry Data and Administrative Health Data for Quality Control? To generate reliable and valid statements on the quality of vascular surgery, both datasets are essential. Administrative data ensure completeness and provide fundamental information about patients, treatments, diagnoses, and patients care pathways. In contrast, the registry offers detailed clinical insights into vascular pathologies and clinical conditions, and provides parameters that are crucial for an accurate analysis of outcome quality. Only the registry enables the formation of more homogeneous patient groups based on specific types of interventions and diagnoses. Treatment and diagnosis codes alone lack the specificity required to capture the vascular surgical perspective on patients without the support of the registry.
Data Matching is the Key
In summary, the Swissvasc registry and administrative health data have both their advantages and limitations. While Swissvasc captures important clinical information, long-term outcome and re-intervention rates are widely lacking but captured by the administrative data (within the canton).
Linking these datasets ensures a more efficient manual data entry process in the registry by capturing only information not already included in the administrative data. This integration enhances the reliability and comprehensiveness of quality analyses in vascular surgery.
Figure 2 shows the two data sources with their limits and advantages.
In a pilot study, Swissvasc and the quality responsible of the Canton of Zurich, analyzed the feasibility of matching the data on a patient level. This matching was successful, with a nearly 100% case match between the two systems. After this matching process, filters to obtain homogenous patient groups were created, mainly using the registry data to get long-term results from individual patients.
Ideally, registries should focus on capturing information that is not already documented in other data sources to avoid redundancy and ensure efficient data collection. To enhance their utility, an automated merging of nationwide health administrative data, including death records, with the registry would be highly beneficial. Such integration would provide a more comprehensive dataset, streamline data analysis, and improve the accuracy and completeness of health outcomes while reducing the burden of manual data entry. A national quality program, where the administrative health data of all vascular units could be matched with registry data would be very helpful to obtain full national coverage.
Conclusion
The Swissvasc registry serves as a valuable instrument for quality assurance, enjoying widespread acceptance across Switzerland. The established close collaboration with regulatory authorities in the Canton of Zurich demonstrates the potential to substantially enhance quality assurance while reducing the administrative burden on hospitals. This quality assurance program in the Canton of Zurich has been well received, and expanding such initiatives on a national scale would be a desirable and beneficial step forward.
- Cronenwett JL, Kraiss LW, Cambria RP. The Society for Vascular Surgery Vascular Quality Initiative. J Vasc Surg. Mai 2012;55(5):1529–37.
- Vascunet Collaboration, Mitchell D, Venermo M, Mani K, Bjorck M, Troeng T, u. a. Quality Improvement in Vascular Surgery: The Role of Comparative Audit and Vascunet. Eur J Vasc Endovasc Surg. 2015;49(1):1–3.
- Wigger P. Swissvasc Registry ist seit 1. Januar 2004 online verfügbar! Schweizerische Ärztezeitung. 2004;85(17):882–3.
- Grima MJ, Ancetti S, Pherwani AD, Gonçalves FB, Budtz-Lilly J, Behrendt CA, u. a. Standards for Quality Improvement Registries on Abdominal Aortic Aneurysm Repair: A Delphi Consensus Report From VASCUNET and International Consortium of Vascular Registries. Eur J Vasc Endovasc Surg. 3. Dezember 2024;S1078-5884(24)01317-0.
- Altreuther M, Grima MJ. International Validation of the Vascular Registry of Switzerland, Swissvasc: A VASCUNET Report. Eur J Vasc Endovasc Surg. 12. Oktober 2024;S1078-5884(24)00889-X.
- Altreuther M, Grima MJ, Lattmann T. International Validation Of Vascular Registries - The VASCUNET Validation Template. Eur J Vasc Endovasc Surg. September 2023;66(3):438–9.
- Swissvasc-Website [Internet]. Verfügbar unter: https://swissvasc.ch/swissvasc-registry.html
- Pherwani AD, Johal AS, Cromwell DA, Boyle JR, Szeberin Z, Venermo M, u. a. Editor’s Choice – Outcomes Following Intact and Ruptured Aneurysm Repair across Nations: Analysis of International Registry Data from the VASCUNET Collaboration 2014 – 2019. European Journal of Vascular and Endovascular Surgery. 1. August 2024;68(2):162–70.
- Venermo M, Mani K, Boyle JR, Eldrup N, Setacci C, Jonsson M, u. a. Editor’s Choice - Sex Related Differences in Indication and Procedural Outcomes of Carotid interventions in VASCUNET. Eur J Vasc Endovasc Surg. Juli 2023;66(1):7–14.
- Grip O, Mani K, Altreuther M, Bastos Gonçalves F, Beiles B, Cassar K, u. a. Contemporary Treatment of Popliteal Artery Aneurysms in 14 Countries: A Vascunet Report. Eur J Vasc Endovasc Surg. November 2020;60(5):721–9.
- Lopez Espada C, Behrendt CA, Mani K, D’Oria M, Lattman T, Khashram M, u. a. Editor’s Choice - The VASCUNExplanT Project: An International Study Assessing Open Surgical Conversion of Failed Non-Infected Endovascular Aortic Aneurysm Repair. Eur J Vasc Endovasc Surg. November 2023;66(5):653–60.
- Meuli L, Lattmann T. [Treatment reality of aortic diseases in Switzerland]. Gefasschirurgie. 2021;26(4):261–9.
- Wanhainen A, Van Herzeele I, Bastos Goncalves F, Bellmunt Montoya S, Berard X, Boyle JR, u. a. Editor’s Choice -- European Society for Vascular Surgery (ESVS) 2024 Clinical Practice Guidelines on the Management of Abdominal Aorto-Iliac Artery Aneurysms. Eur J Vasc Endovasc Surg. Februar 2024;67(2):192–331.
- Meuli L, Kaufmann YL, Lattmann T, Attigah N, Dick F, Mujagic E, u. a. Peri-operative Mortality and Morbidity of Complex Abdominal Aortic Aneurysm Repair in Switzerland: A Swissvasc Report. Eur J Vasc Endovasc Surg. 19. Juni 2024;S1078-5884(24)00540-9.
- Meuli L, Menges AL, Steigmiller K, Kuehnl A, Reutersberg B, Held U, u. a. Hospital incidence and mortality of patients treated for abdominal aortic aneurysms in Switzerland - a secondary analysis of Swiss DRG statistics data. Swiss Med Wkly. 20. Juni 2022;152:w30191.
- Meuli L, Menges AL, Stoklasa K, Steigmiller K, Reutersberg B, Zimmermann A. Inter-Hospital Transfer of Patients With Ruptured Abdominal Aortic Aneurysm in Switzerland. Eur J Vasc Endovasc Surg. April 2023;65(4):484–92.
- Meuli L, Reutersberg B, Risteski P, Dzemali O, Zimmermann A. Hospital incidence, mortality, and gender disparities in patients treated for type A aortic dissections in Switzerland - a secondary data analysis of Swiss DRG statistics. Swiss Med Wkly. 4. Dezember 2023;153:3499.
- Bozalka R, Menges AL, Zimmermann A, Meuli L. Hospital Incidence and Treatment Outcomes of Patients with Aneurysms and Dissections of the Iliac Artery in Switzerland—A Secondary Analysis of Swiss DRG Statistics Data. Journal of Clinical Medicine. Januar 2024;13(8):2267.
- BAG [Internet]. (BAG). Verfügbar unter: https://www.bag.admin.ch/bag/de/home/strategie-und-politik/abstimmungen/volksabstimmung-einheitliche-finanzierung-der-leistungen.html