However, many patients struggled to complete this additional chemotherapy due to reduced tolerance after surgery. This led to the development of total neoadjuvant therapy (TNT), which combines chemotherapy and CRT before surgery.
Studies have shown improved outcomes with TNT compared to the standard approach, including reduced local recurrence rates, increased disease-free survival, and potentially better overall survival. Additionally, TNT with a complete clinical response may allow some patients to avoid surgery altogether through watch-and-wait surveillance, potentially preserving their organs.
This review explores the clinical research that led to the adoption of TNT as the new standard of care for LARC, with a focus on the potential benefits of watch-and-wait surveillance for patients achieving complete responses and with an additional look at the challenges and treatment options for patients with LARC and histopathologically dMMR/MSI-H Status [1].
Introduction
Locally advanced rectal cancer (LARC), encompassing stages II (clinically T3/T4, node-negative) and III (node-positive), represents a significant global burden. Colorectal cancer is a major health concern, ranking as the third most common cancer worldwide. While screening programs improve early detection and reduce mortality in older populations, a concerning rise in LARC diagnoses is observed in younger adults, particularly those under 50 [2].
Treatment for LARC prioritizes preventing cancer spread (locoregional and metastatic recurrence) while maintaining patients' quality of life. The rectum's deep pelvic location makes local recurrences highly problematic. Additionally, a tumor's response to neoadjuvant treatment before surgery is critical for achieving negative surgical margins (no cancer cells within a specific distance of the removed tissue). Positive margins (tumor cells within 2mm) are linked to worse outcomes, including higher local recurrence rates, increased risk of distant spread, and poorer overall survival [3].
Total Neoadjuvant Therapy (TNT) as the Standard of Care for Locally Advanced Rectal Cancer
LARC, defined as tumors invading but not extending beyond the confines of the rectal wall, represents a challenging subset of colorectal cancer, demanding aggressive multimodal treatment approaches to achieve optimal outcomes. Traditionally, the standard treatment regimen for LARC involved CRT followed by surgical resection. However, advancements in systemic chemotherapy and radiotherapy techniques have paved the way for TNT.
TNT combines chemotherapy with CRT before surgery, potentially leading to improved outcomes like complete pathological response (pCR), reduced locoregional recurrence, lower metastatic recurrence, and possibly even better overall survival. [3,4,5].
Benefits of TNT
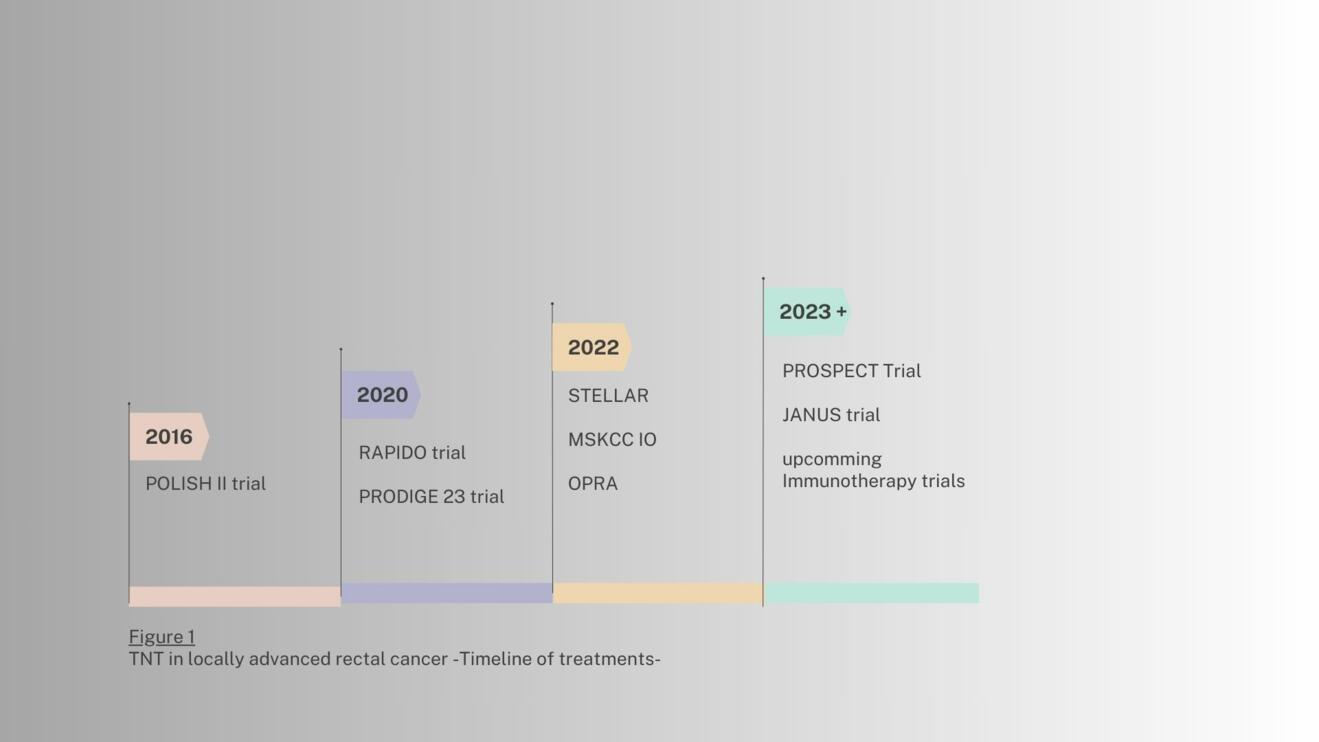
Several studies have examined the value of a TNT in the past (Fig.1). TNT has demonstrated significant advantages over conventional CRT-based strategies in the management of LARC. Key benefits include [1,3].:
– Improved Resectability: TNT has been shown to enhance tumor shrinkage, rendering previously unresectable tumors resectable, leading to higher R0 resection rates, which is associated with improved survival outcomes.
– Enhanced Sphincter Preservation: TNT has demonstrated superior sphincter preservation rates compared to CRT alone, enabling more patients to retain anal function and avoid colostomies.
– Improved Survival Outcomes: Studies have consistently reported superior overall survival (OS) and disease-free survival (DFS) in patients treated with TNT compared to those receiving CRT alone.
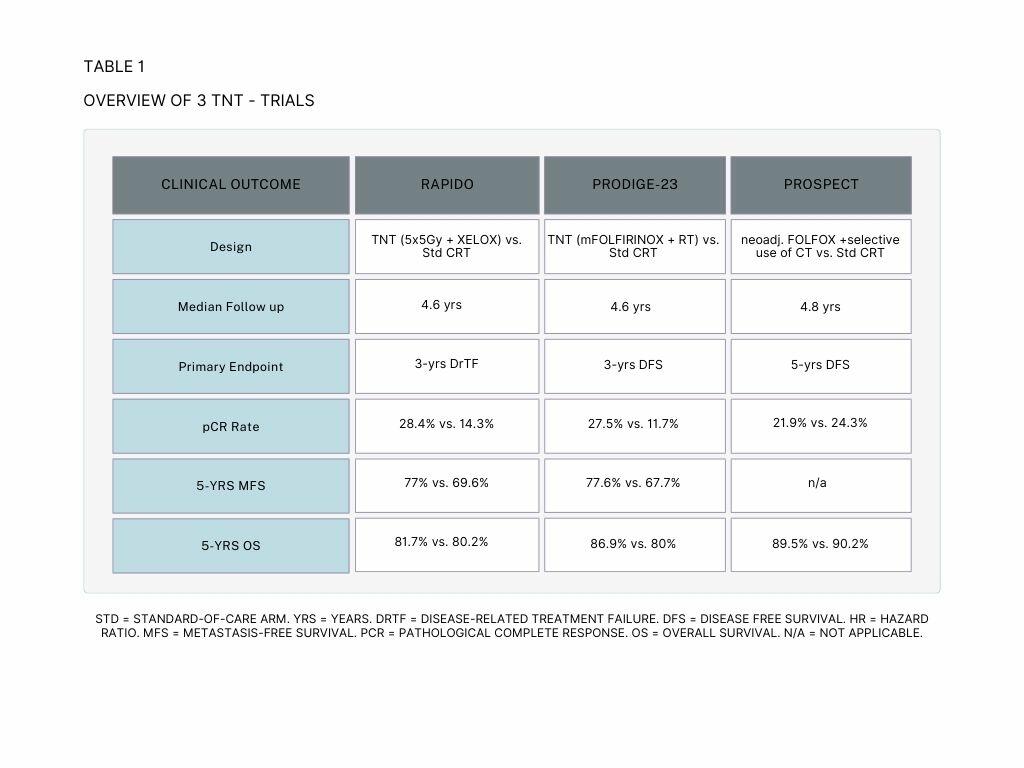
The results of the RAPIDO trial showed that TNT decreased the rate of disease-related treatment failure (DrTF) by 6.7% compared to the standard-of-care treatment. Additionally, TNT doubled the rate of pathological complete response (pCR) and lowered the rate of distant metastases. However, TNT was also associated with higher rates of grade 3 or higher adverse events. An update of the RAPIDO trial showed an increased risk of locoregional recurrence (LRR) in the TNT arm compared to the standard of care arm [Table 1,3,5].
The PRODIGE-23 trial also showed positive results for TNT. Patients receiving TNT exhibited an improved 5-year disease-free survival (DFS) of 7,6%, 6.9% improvement for Overall Survival (OS), 9.9% for Metastasis-Free Survival (MFS) and 5.7% for Cancer Specific Survival compared to the standard-of-care group. Adverse events were similar between the two arms in this trial [4,6].
The PROSPECT trial showed that FOLFOX chemotherapy, followed by radiation with fluorouracil (5FUCRT) only when needed, is just as effective as the standard treatment of 5FUCRT alone for certain patients with locally advanced rectal cancer (LARC) before surgery.
TNT has emerged as one standard of care for LARC, offering significant improvements in resectability, sphincter preservation, and survival outcomes compared to traditional CRT-based approaches. Ongoing research continues to refine TNT regimens and explore novel treatment strategies to further optimize patient outcomes.
Watch-and-Wait vs. Surgical Resection for Patients with Locally Advanced Rectal Cancer and Complete Remission after Neoadjuvant Chemoradiotherapy
The watch-and-wait approach is based on the premise that some patients with cCR may have a truly cured disease and can be spared the morbidity and mortality associated with surgery. This approach has been shown to be safe and effective in several studies, with long-term survival rates comparable to those of patients who undergo surgery. [7,8]:
The potential benefits of the watch-and-wait approach include:
– Avoidance of major surgery and its associated risks
– Preservation of anal function and sexual function
– Improved quality of life
The potential risks of the watch-and-wait approach include:
– The risk of tumor recurrence
– The need for subsequent surgery if the tumor recurs
– The psychological stress of waiting for potential recurrence
Surgical resection remains the standard of care for patients with LARC who do not achieve cCR after CRT. The goal of surgery is to remove all macroscopic tumor and achieve negative margins. Surgery is typically followed by adjuvant chemoradiotherapy to reduce the risk of recurrence [7,8]:
The benefits of surgical resection include:
– The potential for cure
– The ability to remove all macroscopic tumor
– The ability to assess pathologic response to CRT
– The risks of surgical resection include:
– Major surgery and its associated risks
– Potential loss of anal sphincter function and sexual function
— Short-term and long-term morbidity
Selecting the right approach
The decision of whether to pursue a watch-and-wait approach or surgical resection should be made on a case-by-case basis, taking into account the patient's individual characteristics, tumor factors, and personal preferences [7,8].
Factors influencing treatment decision
– Patient's age and overall health
– Patient's preferences
– Pretreatment tumor stage
– Response to CRT
– Presence of high-risk features, such as extramural macrovascular invasion or involvement of the mesorectal fascia
The management of patients with LARC who achieve cCR after CRT is a complex issue. The watch-and-wait approach is a viable option for selected patients and can offer several potential benefits, including the avoidance of major surgery and its associated risks. However, it is important to carefully select patients for this approach and to have a plan in place for close monitoring and subsequent intervention if the tumor recurs. [7,8]:
Challenges and Treatment Options for Patients with Locally Advanced Rectal Cancer and Histopathologically dMMR/MSI-H Status
Patients with dMMR/MSI-H LARC have less benefit from neoadjuvant CRT than those with microsatellite stable (MSS) tumors. Dostarlimab, a programmed death-1 (PD-1) inhibitor, has shown promising results in the treatment of dMMR/MSI-H LARC in clinical trials. [9,10,11]:
Treatment Options
The treatment options for patients with LARC and dMMR/MSI-H status include:
– Dostarlimab: Dostarlimab is a PD-1 inhibitor that has shown promising results in the treatment of dMMR/MSI-H LARC. PD-1 inhibitors work by blocking the PD-1 protein, which helps cancer cells evade the immune system. Dostarlimab has been shown to shrink tumors and improve survival in patients with dMMR/MSI-H LARC.
– Other treatment options: Other treatment options for patients with dMMR/MSI-H LARC include chemotherapy, radiation therapy, and surgery. These treatments may be used alone or in combination with dostarlimab. [9,10]:
Andrea Cercek et al. demonstrated a high degree of sensitivity to single-agent PD-1 blockade in mismatch repair-deficient, locally advanced rectal cancer. Extended follow-up is required to assess response durability but it offers new hope for patients with this challenging disease [11].
Conclusion
This review highlights the evolving treatment landscape for locally advanced rectal cancer (LARC). Total neoadjuvant therapy (TNT) has emerged as the standard of care, offering significant advantages over traditional approaches in terms of resectability, sphincter preservation, and survival outcomes.
However, the management of patients achieving complete remission (cCR) after chemoradiotherapy (CRT) remains a complex issue. The watch-and-wait strategy presents a viable option for selected patients, potentially avoiding major surgery and its associated risks. Careful patient selection and close monitoring are crucial for this approach.
For patients with LARC and dMMR/MSI-H status, who have less benefit from neoadjuvant CRT, Dostarlimab, a PD-1 inhibitor, has shown promising results in clinical trials. Further research is ongoing to refine treatment regimens and explore novel strategies to optimize outcomes for all LARC patients.
- M.L. Conces and A. Mahipa: Adoption of Total Neoadjuvant Therapy in the Treatment of Locally Advanced Rectal Cancer, Curr. Oncol. 2024, 31(1), 366-382
- Siegel, R.L.; Wagle, N.S.; Cercek, A.; Smith, R.A.; Jemal, A. Colorectal cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 233–254
- Bahadoer, R.R.; Dijkstra, E.A.; van Etten, B.; Marijnen, C.A.M.; Putter, H.; Kranenbarg, E.M.; Roodvoets, A.G.H.; Nagtegaal, I.D.; Beets-Tan, R.G.H.; Blomqvist, L.K.; et al. Short-course radiotherapy followed by chemotherapy before total mesorectal excision (TME) versus preoperative chemoradiotherapy, TME, and optional adjuvant chemotherapy in locally advanced rectal cancer (RAPIDO): A randomised, open-label, phase 3 trial. Lancet Oncol. 2021, 22, 29–42
- Conroy, T.; Etienne, P.-L.; Rio, E.; Evesque, L.; Mesgouez-Nebout, N.; Vendrely, V.; Artignan, X.; Bouche, O.; Boileve, A.; Delaye, M.; et al. Total neoadjuvant therapy with mFOLFIRINOX versus preoperative chemoradiation in patients with locally advanced rectal cancer: 7-year results of PRODIGE 23 phase III trial, a UNICANCER GI trial. JCO 2023, 41, LBA3504
- Dijkstra, E.A.; Nilsson, P.J.; Hospers, G.A.P.; Bahadoer, R.R.; Meershoek-Klein Kranenbarg, E.; Roodvoets, A.G.H.; Putter, H.; Berglund, A.; Cervantes, A.; Crolla, R.; et al. Locoregional Failure During and After Short-course Radiotherapy followed by Chemotherapy and Surgery Compared to Long-course Chemoradiotherapy and Surgery—A Five-year Follow-up of the RAPIDO Trial. Ann. Surg. 2023, 278, e766–e772.
- V. Dapra et al. Total Neoadjuvant Treatment for Locally Advanced Rectal Cancer Patients: Where Do We Stand? Int J Mol Sci. 2023 Aug; 24(15): 12159.
- M.J.M. van der Valk et al. Long-term outcomes of clinical complete responders after neoadjuvant treatment for rectal cancer in the International Watch & Wait Database (IWWD): an international multicentre registry study (2018) The Lancet, VOLUME 391, ISSUE 10139, P2537-2545.
- J.B. Yuval et. Al. Watch-and-Wait Management for Rectal Cancer after Clinical Complete Response to Neoadjuvant Therapy (2021) Adv Surg. Sep; 55: 89–107.
- A. Cercek et. al. Phase II, single-arm, open-label study of dostarlimab monotherapy in previously untreated patients with stage II/III dMMR/MSI-H locally advanced rectal cancer. 2023 Journal of Clinical Oncology Volume 41, Number 16_supp l
- R. Yang et al. Locally advanced rectal cancer with dMMR/MSI-H may be excused from surgery after neoadjuvant anti-PD-1 monotherapy: a multiple-center, cohort study (2023) Front Immunol. Jun 27:14:1182299.
- A. Cercek et. al, PD-1 Blockade in Mismatch Repair–Deficient, Locally Advanced Rectal Cancer (2022) N Engl J Med 2022;386:2363-2376