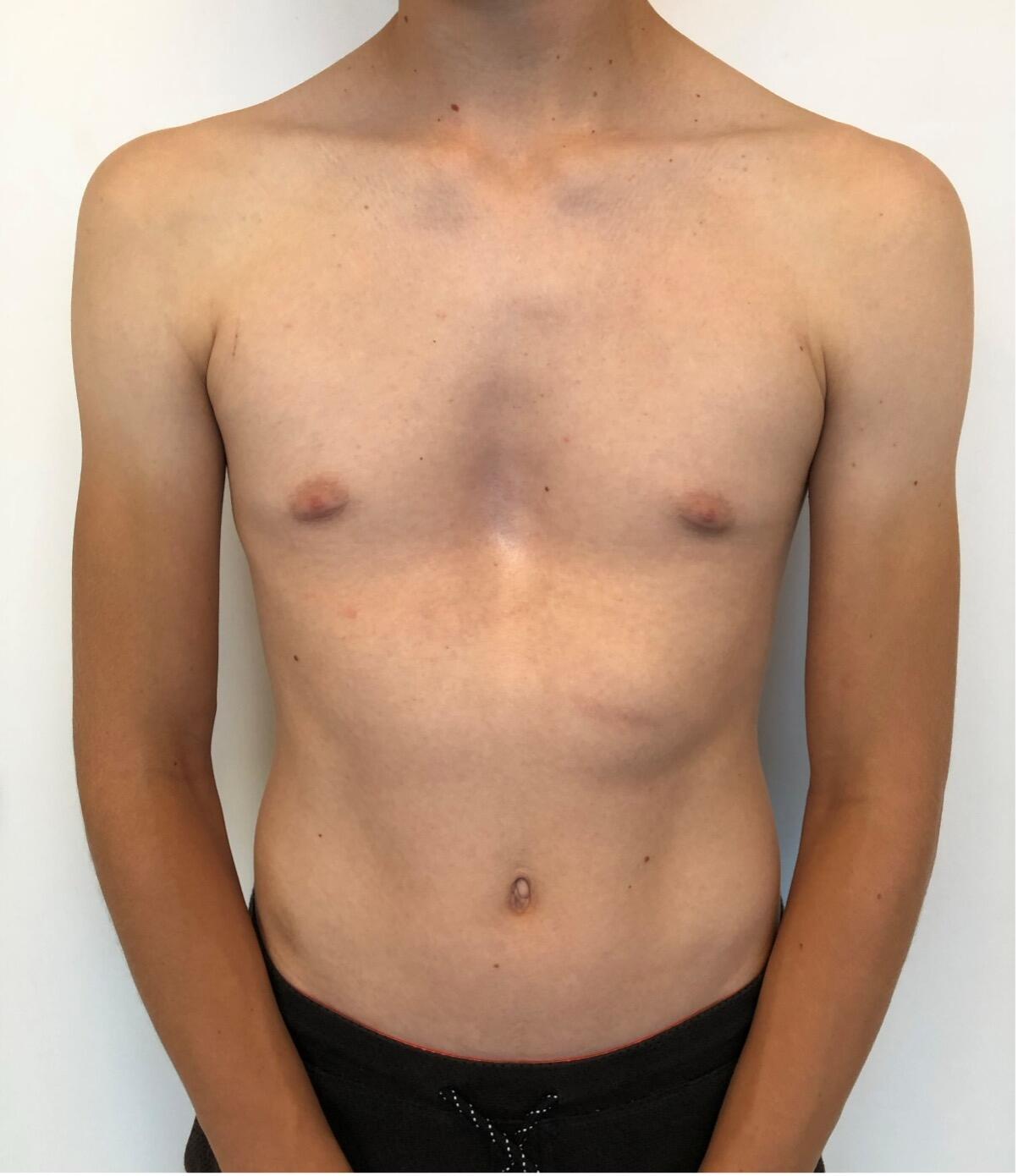
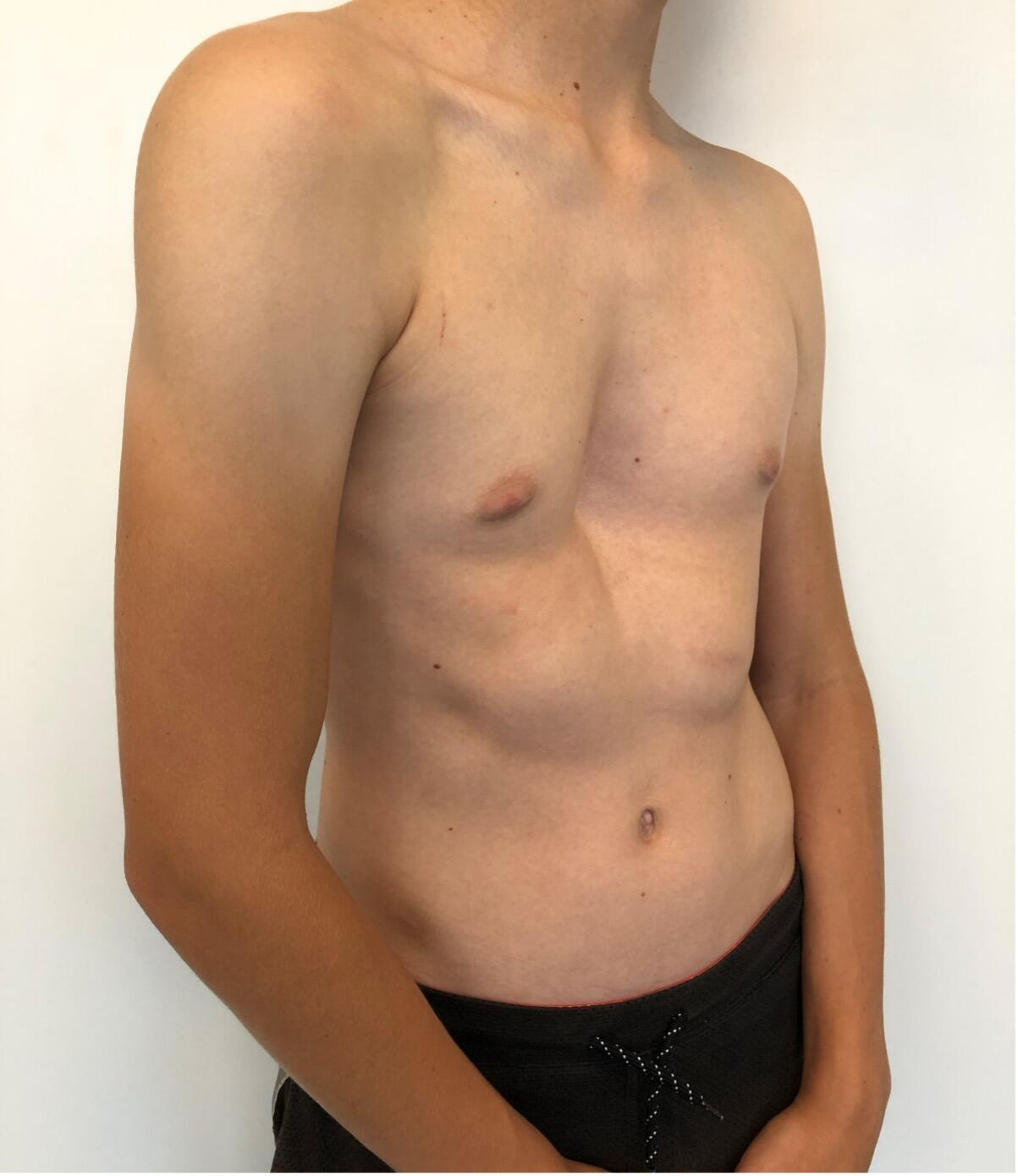
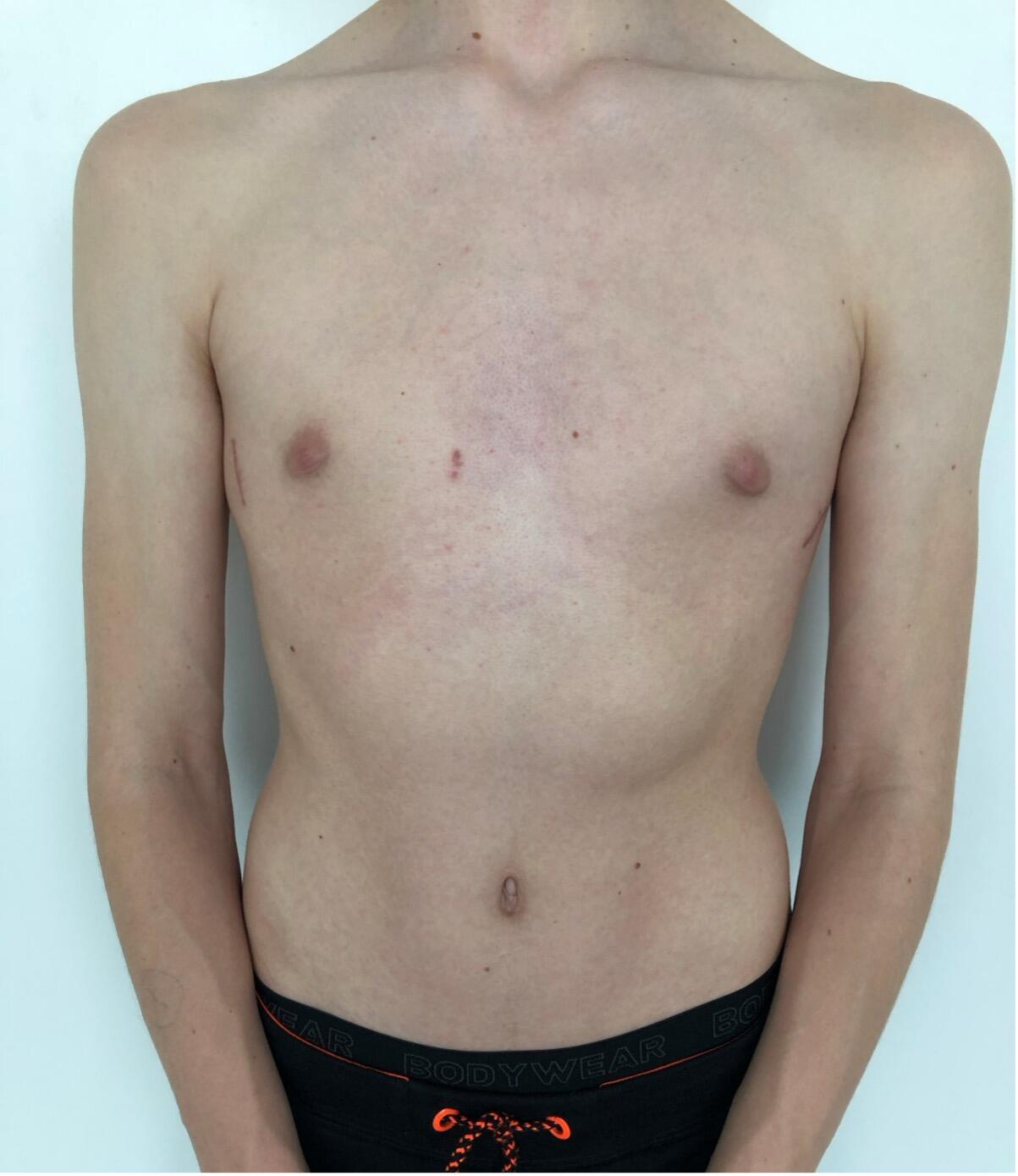
The most common type of chest wall deformity with an incidence of one in 300 – 400 live births[i] is the “funnel” or “sunken” chest also known as pectus excavatum (PE). (Figures 1a and 1b)
There is a male to female preponderance of roughly 4:1. PE is thought to be the result of an abnormal growth of costal cartilage, but the exact cause remains unclear. Some cases of PE have been linked to a family history suggesting a possible genetic predisposition. It is often present at birth and commonly exacerbates during puberty. Most are isolated deformities[ii] but PE is also associated with systemic connective tissue disorders including Marfan syndrome, Ehlers-Danlos, Loeys-Dietz and osteogenesis imperfecta[iii] [iv] In the past, PE was considered mainly a cosmetic problem albeit one with a major psychological impact including a poor self-image and social anxiety, feelings of inferiority and even depression that are potentially devastating to these patients resulting in a significant reduction of quality of life[v] [vi]. Today, however we know that it may also have a myriad of associated symptoms and adverse effects depending on the severity of the deformation. These include impaired cardiopulmonary performance like shortness of breath on exertion, loss of endurance, fatigue, chest pain, palpitations and tachycardia and respiratory infections[vii] [viii].
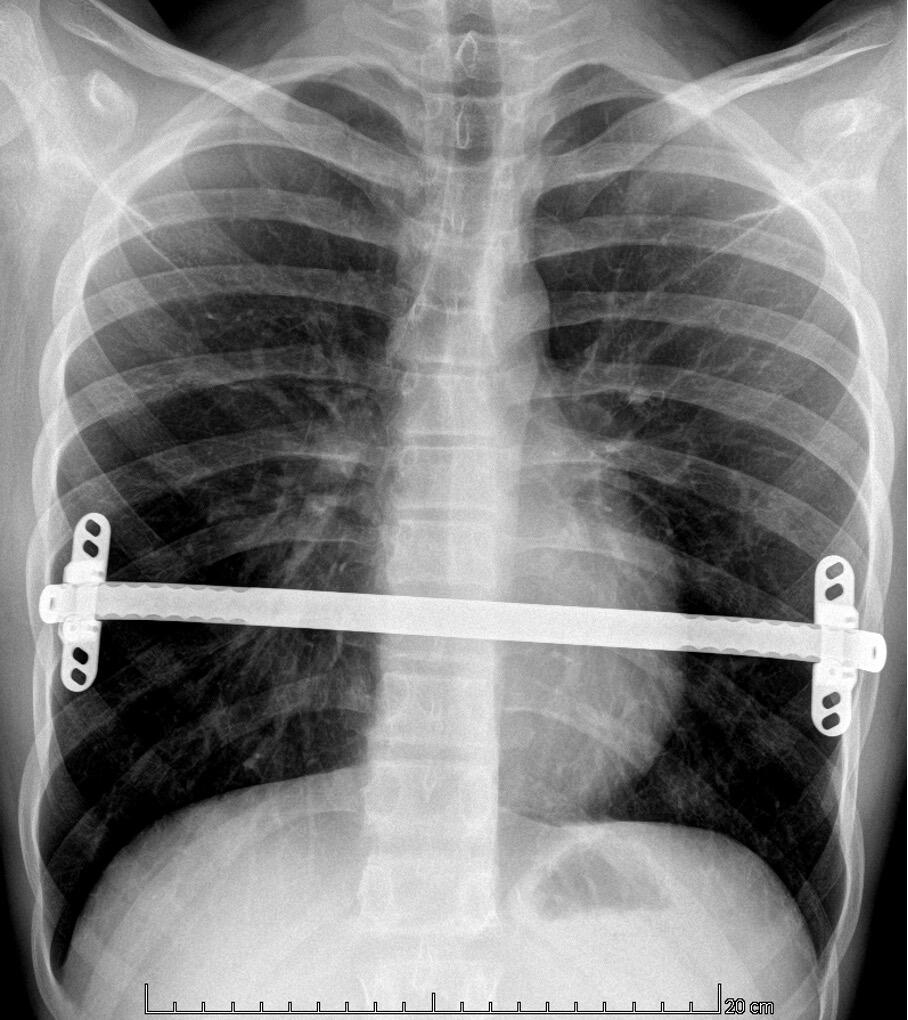
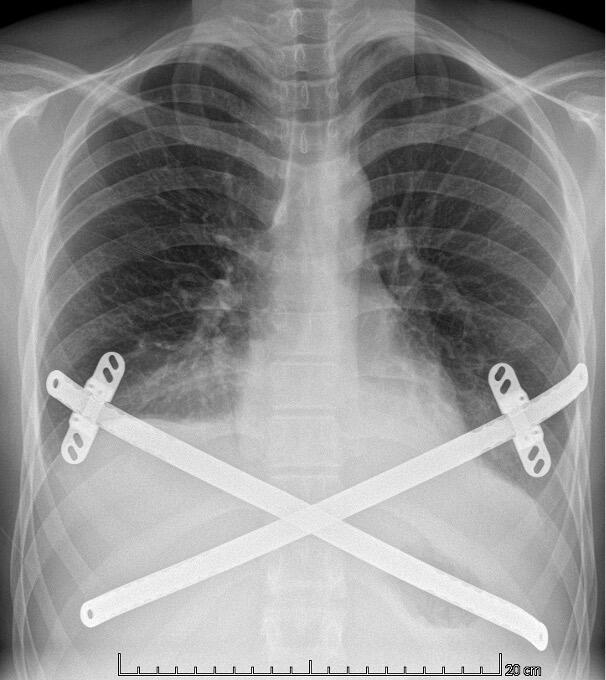
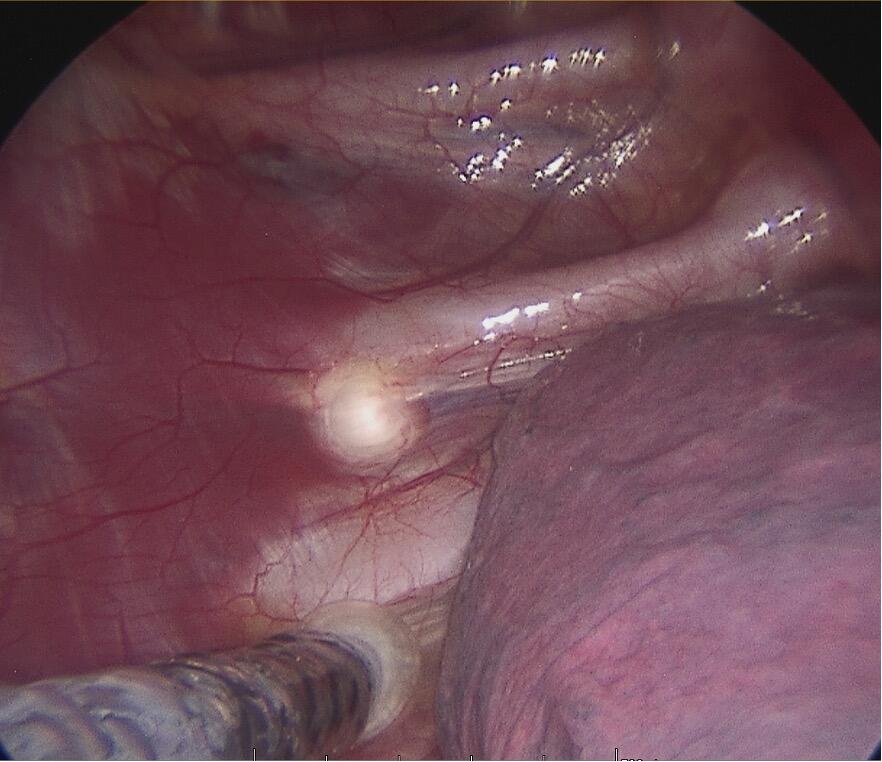
Diagnosing PE typically involves a combination of clinical evaluation and imaging studies. Treatment options are plentiful varying from the non-invasive Vacuum Bell[ix] to different surgical techniques such as custom-made silicone implants[x], the “Pectus Up”[xi] and the “modified” Ravitch procedures[xii]. However, the by far most frequently used technique today, is the thoracoscopically assisted, Nuss procedure or Minimal Invasive Repair of Pectus Excavatum, (MIRPE)[xiii]. This involves placing one or more pre-formed curved metal bars retro-sternally to reshape the chest wall and improve its appearance. The bar or bars themselves are inserted through small incisions made laterally on the chest and are then rotated through 180° to elevate the sternum and push the chest wall outward. The original Nuss procedure described a single bar insertion but since its introduction in 1998 there have been many modifications. (Figures 2 and 3) There is a tendency nowadays to place multiple bars especially in the adult population.
Cryoanalgesia
In experienced hands the Nuss procedure is a safe and very efficient method for correcting a large proportion of these chest wall malformations. (Figure 4) The advantages of this technique over the “modified” Ravitch procedure were unfortunately not accompanied by a decrease in postoperative pain nor length of stay (LOS). The procedure is characterised by intense pain and postoperative pain management is a significant challenge. In an attempt to mitigate the pain, multiple, multi-modal anaesthetic regimes have been proposed including epidural thoracic anaesthesia, intravenous, patient-controlled analgesia (PCA), local or regional blocks and indwelling chest wall catheters but no regime is clearly better than another.
In 1974 Nelson was the first to describe the technique of intraoperative nerve freezing to prevent post thoracotomy pain[xiv]. It was not until 2016 when Kim introduced cryoanalgesia to the Nuss procedure[xv] using the first commercially available cryoablation probe (CryoICE®, AtriCure, Mason, Ohio, USA).
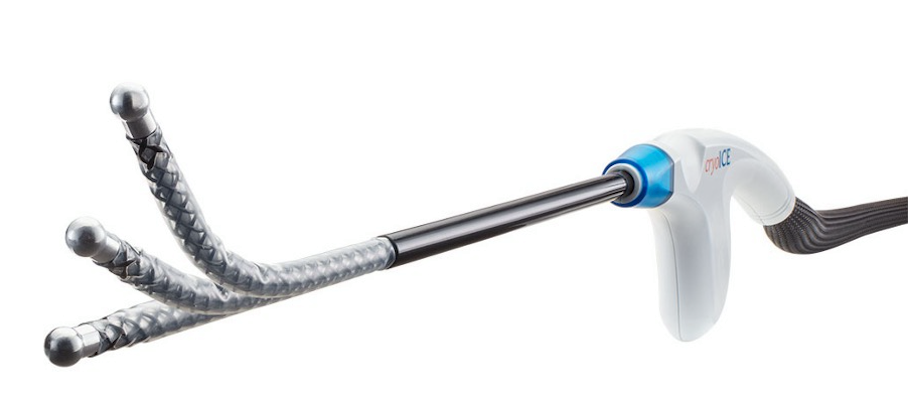
Cryoanalgesia is a form of regional anaesthesia in which nerve conduction is temporarily blocked by freezing the target nerve to roughly -60°C for a period of two minutes, providing long-term pain relief. Figure 5. This temporary injury to the nerve is called axonotmesis whereby only the axon itself and its myelin sheath are damaged but the endoneurium, perineurium and epineurium remain intact. After Wallerian degeneration distal to the point of freezing, the axon regenerates along its original nerve path and the sensation returns to normal. AtriCure cryoSPHERE® (AtriCure, Mason, Ohio, USA), Figure 6 is to date, the only commercially available system in Switzerland for intraoperative cryoanalgesia.
Over the years, cryoanalgesia has been compared to other pain management regimes in patients undergoing a Nuss procedure for a PE, focussing on patient outcomes like length of surgical intervention, postoperative pain scores, LOS, opioid use, complications, and costs. A systematic review published 2023 by Eldredge and McMahon[xvi] found that cryoanalgesia when compared with other analgesic strategies was associated with a significant decrease in LOS, significantly lower opioid use, lower costs, and less complications. Akinboro et al even found it possible to discharge 65% of patients undergoing MIRPE on the day of surgery, when it was combined with concomitant cryoanalgesia and a bilateral, single-shot paravertebral block[xvii].
The complications associated with cryoanalgesia have been carefully studied. In the systematic review by Scott Eldregde and McMahonxvi, published in 2023, found that in the articles where complications were reported, the complication rate was significantly less, or no difference was found between cryoanalgesia and non-cryoanalgesia cohorts. Persistent sensory loss and neuropathic pain is also of concern especially in the adult population. However, in a study published by Zobel in 2020[xviii] where patients below the age of 21 were compared to patients above 21, it was demonstrated that the younger patients had a significantly faster recovery time to normal, preoperative chest wall sensation and that neuropathic pain was not encountered. This was corroborated by a recently published study by van Braak[xix].
The Geneva Experience
Since 2015 the University Hospitals in Geneva (HUG), the Paediatric Surgical Department together with the department of Paediatric Pulmonology, Paediatric Orthopaedics and the Division of Genetic Medicine runs multidisciplinary consultations for patients with chest wall malformations. Patients with a PE are offered a myriad of treatment options. These include the treatment by Vacuum Bell, lipofilling, custom-made 3D silicone implants, the modified Ravitch procedure and of course MIRPE. The department of Paediatric Surgery performs between 12 and 15 MIRPE procedures, annually. All patients undergo a strict pre-treatment work-up which includes pulmonary and cardiac evaluation, a cardiac MRI and a low dose 2D/3D imaging of the vertebral column (EOS imaging). Those requiring surgery will also have a chest CT scan with 3D reconstruction to help in the preoperative planning. Prior to October 2022, patients undergoing a MIRPE procedure all received multi-modal anaesthetic regimes including an epidural which was switched to a PCA at day 5 postoperatively, Tizanidine, a muscle relaxant, and all had a urinary catheter inserted at the time of surgery. From October 2022, we, as the first in Switzerland, started using cryoanalgesia (AtriCure cryoSPHERE® supplied by Mussler Medical, Morges, Switzerland, Figure 6) intraoperatively for patients undergoing a MIRPE procedure.
After a few minor changes to our initial protocol, all patients are now operated under general anaesthesia with a single-lumen endotracheal tube. They undergo a bilateral erector spinae plane block and before bar placement, thoracoscopic, bilateral cryoanalgesia is performed, from T3 to T8. In general, cryoanalgesia is not done above T3 to minimise the risk of Horner syndrome and not below T8 to prevent rectus abdominis paralysis. Postoperatively, tizanidine, Nonsteroidal Anti-Inflammatory Drugs (NSAIDS) and paracetamol are prescribed. Patients do not receive morphine nor a urinary catheter.
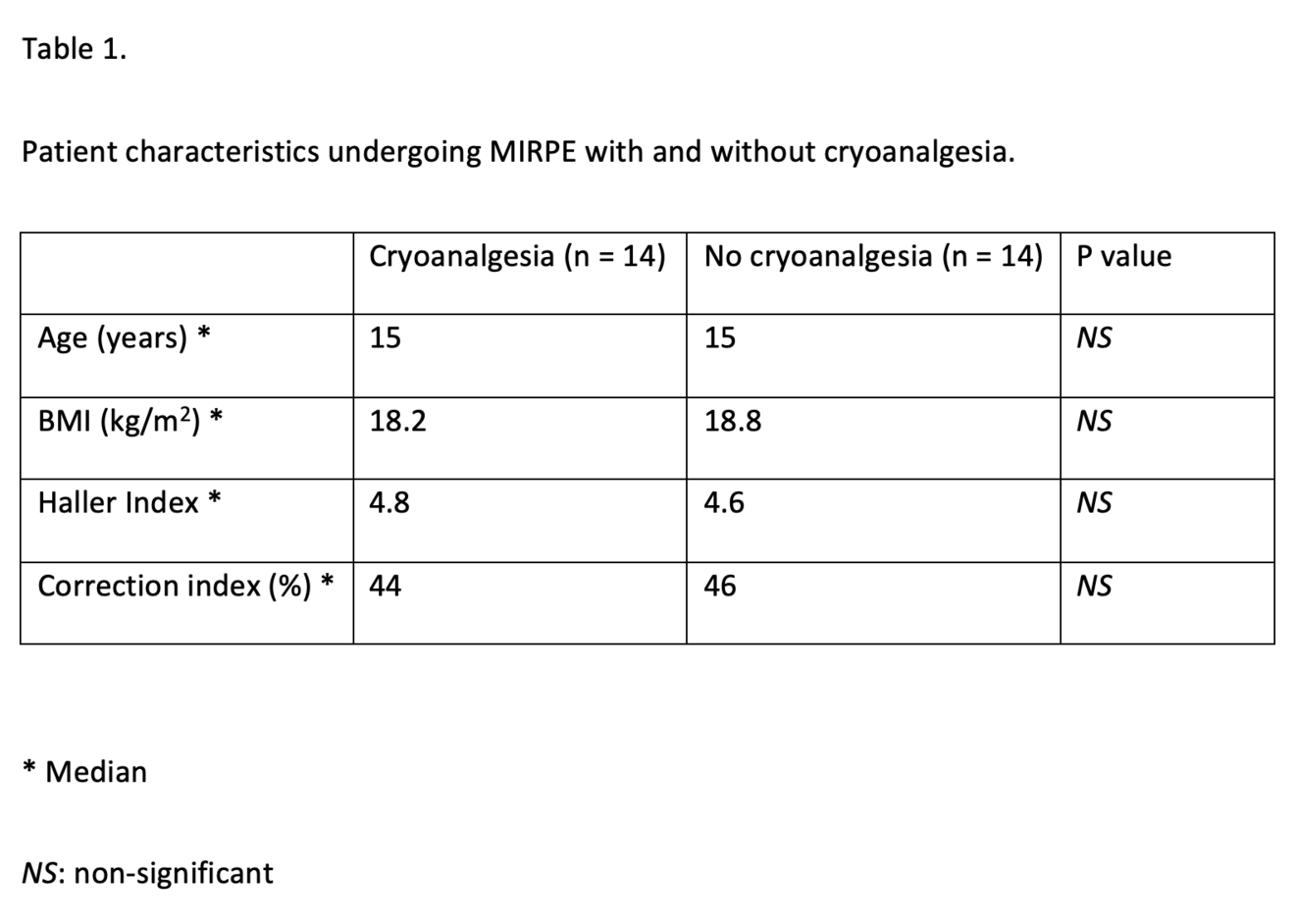
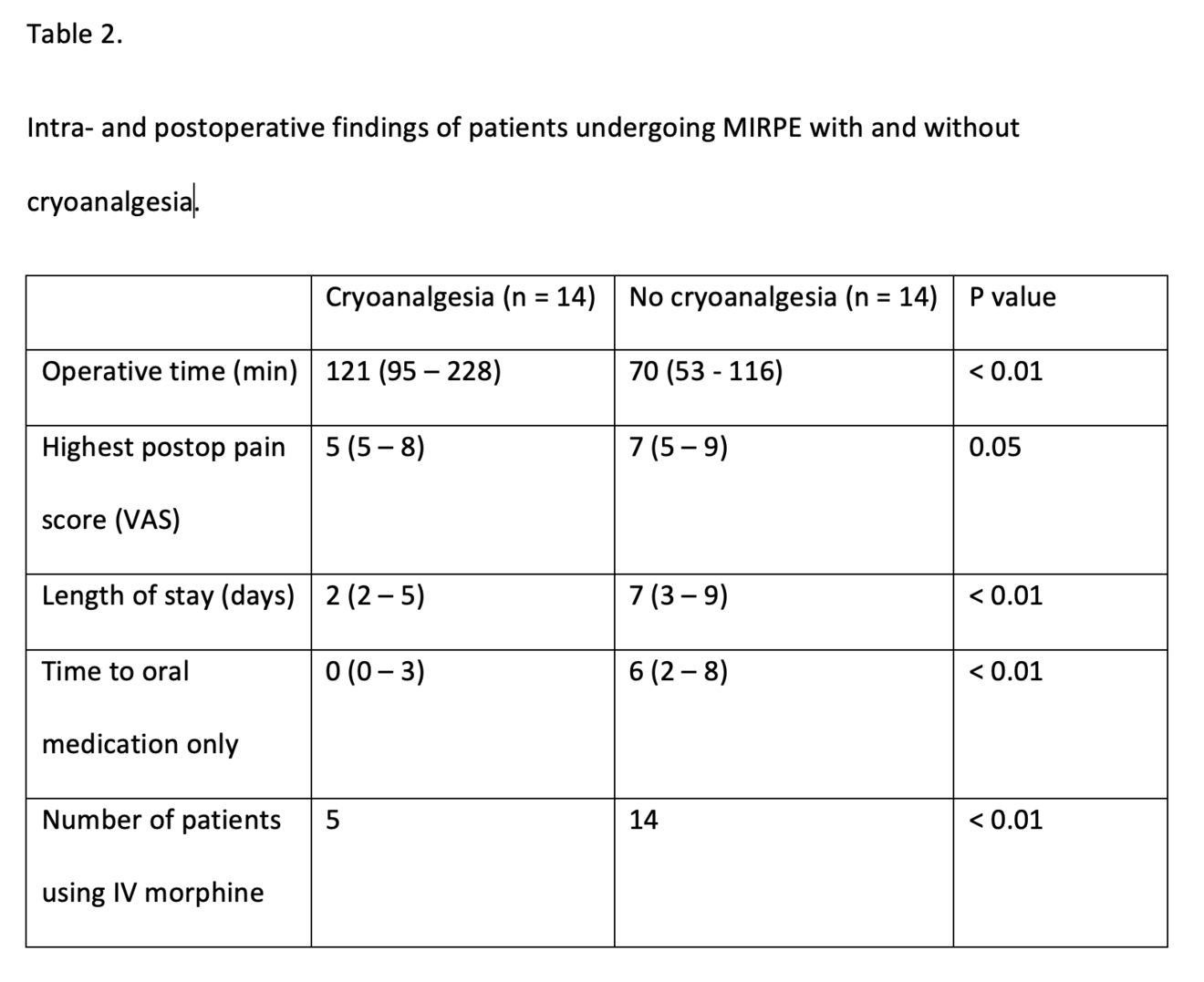
We compared all our patients undergoing a MIRPE procedure with cryoanalgesia with a historic cohort of 14 patients who underwent the same procedure with the standard multi-modal anaesthetic regime at the time. Both groups of patients were similar in their baseline characteristics, Table 1.
Patients in both groups received a similar number of bars. The cross-bar technique, sometimes used for patients with a deep PE or those patients with concomitant rib-flaring was used when indicated from 2022 onwards. Figure 3. Operative time was significantly longer for those undergoing a MIRPE with cryoanalgesia than those without. On average the operation took 50 minutes longer (p < 0.01). Once having passed our learning curve bilateral cryoanalgesia adds on average 40 minutes to the procedure while this was around 75 minutes in our first seven cases. The LOS has been significantly reduced after the implementation of cryoanalgesia. This has been reduced from 7 days of hospitalisation for those patients undergoing MIRPE without cryoanalgesia to 2 days for those with (p < 0.01). The time to exclusive oral pain medication has been shortened from 6 to 0 days (p < 0.01). Postoperative opioid use, which is often looked at when multimodal anaesthetic regimes are compared to cryoanalgesia can be defined in different ways. As we adapted our protocol during this study, we can only say that our first five patients undergoing MIRPE in combination with cryoanalgesia received intravenous morphine in accordance with the protocol at the time and the following nine, did not. All patients in our control group who were operated without cryoanalgesia received morphine intravenously. (See Table 2)
In our cohort, pain sensation of the chest returned to normal in all our patients between 6 weeks and 6 months post-surgery. It bridged the difficult period very efficiently when patients complain of chest- and back pain in the first few months post MIRPE.
One patient who underwent MIRPE with a cross-bar technique developed a pleural effusion and required thoracocentesis for symptom relief. It is difficult to say whether this was due to the cryoanalgesia or the cross-bar technique as both are associated with an increased risk of developing a pleural effusion[xx]. One patient required a urinary catheter on day one post-surgery for a urinary retention. One patient was readmitted for pain management at three weeks after surgery after having stopped taking pain medication a week after he had left the hospital.
Conclusion
Since cryoanalgesia was introduced as part of the arsenal for pain-relief after MIRPE, its popularity worldwide has gained exponentially. As MIRPE, today, is the gold standard, the procedure against which all other techniques for the correction of PE must be compared against; all multi-modal anaesthetic regimes developed to combat with the postoperative pain after MIRPE must be compared with cryoanalgesia. With this audit of our first 14 patients treated for pectus excavatum by MIRPE in combination of cryoanalgesia, we have shown its beneficial effect in terms of LOS, postoperative pain control and opioid use. At the HUG, we would have great ethical difficulties going back to the MIRPE, without cryoanalgesia.
- Chung CS, Myrianthopoulos NC. Factors affecting risks of congenital malformations. I. Analysis of epidemiologic factors in congenital malformations. Report from the Collaborative Perinatal Project. Birth Defects Orig Artic Ser. 1975;11(10):1-22. PMID: 130944.
- Fokin AA, Steuerwald NM, Ahrens WA, Allen KE. Anatomical, histologic, and genetic characteristics of congenital chest wall deformities. Semin Thorac Cardiovasc Surg. 2009 Spring;21(1):44-57. doi: 10.1053/j.semtcvs.2009.03.001. PMID: 19632563.
- Behr CA, Denning NL, Kallis MP, Maloney C, Soffer SZ, Romano-Adesman A, Hong AR. The incidence of Marfan syndrome and cardiac anomalies in patients presenting with pectus deformities. J Pediatr Surg. 2019 Sep;54(9):1926-1928. doi: 10.1016/j.jpedsurg.2018.11.017. Epub 2018 Dec 27. PMID: 30686517.
- Loeys BL, Dietz HC. Loeys-Dietz Syndrome. 2008 Feb 28 [updated 2018 Mar 1]. In: Adam MP, Feldman J, Mirzaa GM, Pagon RA, Wallace SE, Bean LJH, Gripp KW, Amemiya A, editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993–2024. PMID: 20301312.
- Kelly RE Jr, Cash TF, Shamberger RC, Mitchell KK, Mellins RB, Lawson ML, Oldham K, Azizkhan RG, Hebra AV, Nuss D, Goretsky MJ, Sharp RJ, Holcomb GW 3rd, Shim WK, Megison SM, Moss RL, Fecteau AH, Colombani PM, Bagley T, Quinn A, Moskowitz AB. Surgical repair of pectus excavatum markedly improves body image and perceived ability for physical activity: multicenter study. Pediatrics. 2008 Dec;122(6):1218-22. doi: 10.1542/peds.2007-2723. PMID: 19047237.
- Zuidema WP, Oosterhuis JWA, Zijp GW, van der Heide SM, van der Steeg AFW, van Heurn LWE. Early Consequences of Pectus Excavatum Surgery on Self-Esteem and General Quality of Life. World J Surg. 2018 Aug;42(8):2502-2506. doi: 10.1007/s00268-018-4526-9. PMID: 29411068; PMCID: PMC6060811.
- Malek MH, Fonkalsrud EW, Cooper CB. Ventilatory and cardiovascular responses to exercise in patients with pectus excavatum. Chest. 2003 Sep;124(3):870-82. doi: 10.1378/chest.124.3.870. PMID: 12970011.
- Maagaard M, Heiberg J. Improved cardiac function and exercise capacity following correction of pectus excavatum: a review of current literature. Ann Cardiothorac Surg. 2016 Sep;5(5):485-492. doi: 10.21037/acs.2016.09.03. PMID: 27747182; PMCID: PMC5056930.
- Schier F, Bahr M, Klobe E. The vacuum chest wall lifter: an innovative, non-surgical addition to the management of pectus excavatum. J Pediatr Surg. 2004.2004.11.033
- Chavoin JP, Grolleau JL, Moreno B, Brunello J, André A, Dahan M, Garrido I, Chaput B. Correction of Pectus Excavatum by Custom-Made Silicone Implants: Contribution of Computer-Aided Design Reconstruction. A 20-Year Experience and 401 Cases. Plast Reconstr Surg. 2016 May;137(5):860e-871e.
- Bardají C, Cassou L. Taulinoplasty: the traction technique-a new extrathoracic repair for pectus excavatum. Ann Cardiothorac Surg. 2016 Sep;5(5):519-522. doi: 10.21037/acs.2016.09.07. PMID: 27747186; PMCID: PMC5056940.
- Ravitch MM. New trends in pediatric surgery; pectus excavatum, esophageal atresia, intussusception, Hirschsprung's disease. Surg Clin North Am. 1949 Oct;29(4):1535-50. doi: 10.1016/s0039-6109(16)32844-4. PMID: 18141306.
- Nuss D, Kelly RE Jr, Croitoru DP, Katz ME. A 10-year review of a minimally invasive technique for the correction of pectus excavatum. J Pediatr Surg. 1998 Apr;33(4):545-52. doi: 10.1016/s0022-3468(98)90314-1. PMID: 9574749.
- Nelson KM, Vincent RG, Bourke RS, Smith DE, Blakeley WR, Kaplan RJ, Pollay M. Intraoperative intercostal nerve freezing to prevent postthoracotomy pain. Ann Thorac Surg. 1974 Sep;18(3):280-5. doi: 10.1016/s0003-4975(10)64357-3. PMID: 4413968.
- Kim S, Idowu O, Palmer B, Lee SH. Use of transthoracic cryoanalgesia during the Nuss procedure. J Thorac Cardiovasc Surg. 2016 Mar;151(3):887-888. doi: 10.1016/j.jtcvs.2015.09.110. PMID: 26896363.
- Eldredge RS, McMahon L. Intercostal nerve cryoablation therapy for the repair of pectus excavatum: a systematic review. Front Surg. 2023 Aug 24;10:1235120. doi: 10.3389/fsurg.2023.1235120. PMID: 37693640; PMCID: PMC10484532.
- Akinboro S, John R, Reyna T, Davis R, Ayoub C, Sangster R, Kim J, Nguyen H, Moreno C, Guner Y, Goodman L, Yu PT, Morphew T, Kabeer M. A pilot study of multi-modal pain management for same-day discharge after minimally invasive repair of pectus excavatum (Nuss procedure) in children. Pediatr Surg Int 39, 159 (2023)
- Zobel, M.J., Ewbank, C., Mora, R. et al. The incidence of neuropathic pain after intercostal cryoablation during the Nuss procedure. Pediatr Surg Int 36, 317-324(2020) https://doi.org/10.1007/s00383-019-04602-1
- van Braak H, de Beer S, de Jong J R et al. Intercostal nerve cryoablation or epidural analgesia for multimodal pain management after the Nuss procedure: a cohort study. European Journal of Pediatric Surgery 2024. doi: 10.1055/a-2249-7588
- Sayan B, Bekiroglu N, Yuksel M. Pectus cross bars increase hospital readmission rates due to serous pleural effusion. Gen Thorac Cardiovasc Surg. 2022 Apr;70(4):352-358. doi: 10.1007/s11748-021-01732-z. Epub 2021 Nov 16. PMID: 34784003.