Excessive perioperative fluid administration contributes to interstitial edema, impaired organ function, and increased complications. This review details the mechanisms of fluid accumulation, its impacts on outcomes, and strategies to identify the optimal timing for de-resuscitation. We discuss the ROSE model phases and review pharmacologic, extracorporeal, and mechanical modalities for fluid removal. By integrating clinical triggers with dynamic assessments, a de-resuscitation may restore normovolemia, reduce morbidity, and accelerate recovery.
Introduction
Perioperative fluid administration remains a cornerstone of hemodynamic management in major surgery, aiming to preserve intravascular volume, optimize cardiac preload, and maintain tissue perfusion (1-3). Historically, liberal crystalloid and colloid boluses were employed to counteract anesthetic-induced vasodilation and surgical blood loss. However, mounting evidence now links excessive fluid loading to interstitial edema, impaired organ function, and increased morbidity and mortality (1, 2, 4). In a meta-analysis, Messmer et al. demonstrated a dose-dependent relationship between cumulative positive fluid balance and mortality in critically ill patients (1). This phenomenon, called “fluid accumulation syndrome,” highlights the cascade of endothelial injury, capillary leak, and tissue edema (2). Investigations reinforced these concerns in septic shock, showing that over-resuscitation prolongs mechanical ventilation, increases ICU length of stay, and raises the incidence of acute kidney injury (3). In elective surgical cohorts, Heming et al. emphasize that both hypovolemia and fluid accumulation are harmful, advocating a zero-balance, individualized approach guided by dynamic assessments rather than fixed volumes (5). Within this evolving paradigm, de-resuscitation strategies—active removal of accumulated fluid once stabilization is achieved—have emerged as essential interventions to restore normovolemia, reduce edema, and accelerate recovery in critically ill patients.
Pathophysiology of Fluid Accumulation in Major Surgery
Major surgical interventions provoke profound shifts in fluid compartments through neuro-hormonal, inflammatory, and mechanical mechanisms. Intraoperatively, blood loss, anesthetic-induced vasodilation, and redistribution of plasma volume reduce effective circulating volume, prompting fluid administration to maintain perfusion (6-8). Simultaneously, the surgical stress response activates the sympathetic nervous system, the renin–angiotensin–aldosterone axis, and antidiuretic hormone release, promoting renal sodium and water retention even before exogenous fluids are infused (9). In addition, surgical trauma triggers systemic inflammation: cytokines such as TNF-α and IL-1β degrade the endothelial glycocalyx—a proteoglycan-rich layer that regulates permeability (10-12). According to the revised Starling principle, the glycocalyx maintains the oncotic gradient that prevents fluid extravasation; once disrupted, both crystalloids and colloids leak into the interstitium, raising interstitial oncotic pressure and driving edema formation (7, 13). Heming et al. note that inappropriate timing of fluid administration and lack of individualized targets exacerbate this process, underscoring that fluid accumulation reflects context as much as the actual infusion volume (5).
A further factor that needs to be kept in mind is that lymphatic drainage is also impaired (8). Inflammatory mediators inhibit lymphatic contractility via NF-κB–iNOS signaling, thus reducing interstitial fluid clearance (8). Concurrently, fibroblast activation remodels the extracellular matrix, increasing its hydraulic conductivity and facilitating rapid fluid sequestration (7). This self-perpetuating cycle of capillary leak, edema, and organ dysfunction underscores the need for timely fluid removal once initial resuscitation goals are met.
Sources of Fluid Input
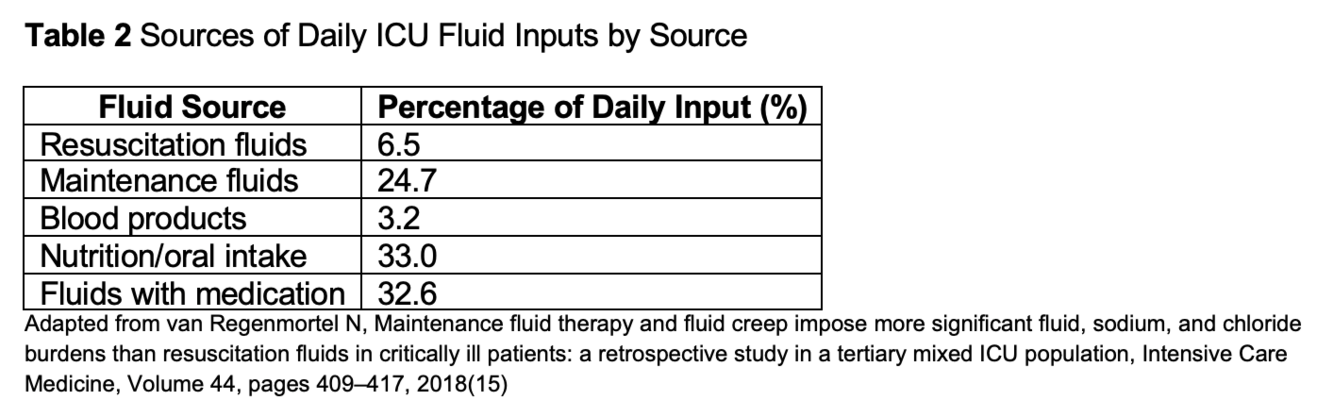
Understanding the full spectrum of fluid sources is critical to tailoring perioperative management and minimizing insidious fluid creep. Exogenous fluid inputs extend far beyond bolus crystalloids and colloids; they include maintenance infusions, medication diluents, blood products, enteral and parenteral nutrition, irrigation solutions, and circuit prime volumes, also see table 2.
Bolus and maintenance crystalloid solutions—normal saline, lactated Ringer’s, or balanced solutions—constitute the backbone of intraoperative volume support. Although essential for correcting hypovolemia, they rapidly distribute to the interstitial space, particularly when the glycocalyx is disrupted (13). Colloids (e.g., albumin, hydroxyethyl starches) theoretically remain intravascular longer but also extravasate in the presence of systemic inflammation, contributing to edema (11).
Blood components—packed red blood cells, plasma, and platelets—deliver volume alongside oxygen-carrying capacity and coagulation factors. However, additive preservative solutions and transfusion-related fluid load can be underestimated, especially when assessing cumulative balance (14).
Medication carrier solutions (creep fluids) are frequently overlooked source of fluid input. Continuous infusions of vasopressors, sedatives, antibiotics, and analgesics often use normal saline or dextrose as carriers; combined flushes for central line maintenance add further fluid volume. A study quantifying iatrogenic infusion fluid estimated that medication diluents accounted for up to 30% of daily fluid input in ICU patients (15).
Nutritional support via enteral or parenteral routes represents another substantial source. Standard continuous enteral feeds can deliver 500–1,000 mL/day of free water, while parenteral nutrition regimens may exceed 2,000 mL/day—including lipid emulsions, amino acid solutions, and dextrose carriers (16). Concentrated formulations and bolus delivery can mitigate fluid load, but coordination with nutrition teams is vital to balance caloric goals against volume constraints (17).
Irrigation fluids used for wound lavage, endoscopy, or joint arthroscopy are often fluid losses, yet residual irrigation can contribute several hundred milliliters per case when retention occurs in tissue planes. Similarly, cardiopulmonary bypass prime volumes and hemofiltration circuits introduce large saline boluses; despite ultrafiltration during bypass, net positive balance frequently persists post-operatively (18).
Finally, procedure-related factors such as third-space sequestration and ongoing capillary leak amplify the impact of every milliliter administered. Negative fluid balances require diligent accounting of these sources, otherwise hidden inputs will undermine any de-resuscitation efforts. Implementing fluid stewardship protocols—enumerating the four D’s (Drug, Dose, Duration, De-escalation)—can help clinicians systematically audit all fluid entries and exits, ensuring every source is recognized and managed appropriately (4, 9).
Impacts of Fluid Accumulation on Surgical Outcomes
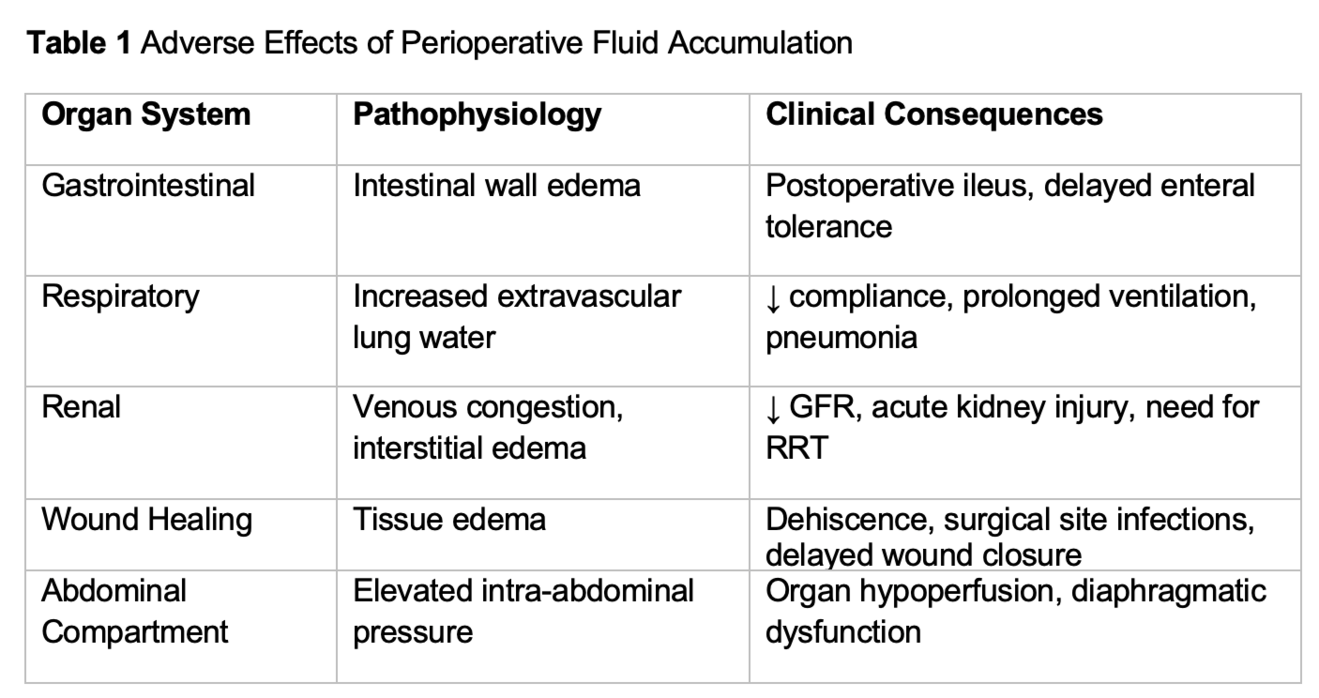
Excessive perioperative fluid accumulation has been consistently associated with a range of adverse surgical outcomes. In abdominal surgery, fluid accumulation contributes to intestinal wall edema, which impairs peristalsis and delays the return of gastrointestinal function. This phenomenon has been linked to prolonged postoperative ileus and delayed tolerance of enteral nutrition, both of which extend hospital stay and increase complication rates (2, 5) (table 1).
Pulmonary complications are also more frequent in patients with fluid accumulation. Increased extravascular lung water impairs gas exchange and reduces pulmonary compliance, leading to longer durations of mechanical ventilation and higher rates of postoperative pulmonary edema and pneumonia (19, 20). In a randomized trial of conservative versus liberal fluid strategies, patients managed conservatively (=less fluids) had improved oxygenation and were liberated from ventilatory support earlier (19).
Renal outcomes are similarly affected. Venous congestion and interstitial renal edema reduce glomerular filtration and contribute to the development of acute kidney injury (AKI). Observational studies have shown that positive fluid balance is an independent predictor of AKI and the need for renal replacement therapy in surgical and critically ill patients (21, 22).
Wound healing is impaired by tissue edema, which increases the diffusion distance for oxygen and nutrients. This has been associated with higher rates of wound dehiscence, surgical site infection, and delayed primary closure, particularly in patients undergoing major abdominal or cardiac surgery (2, 23).
Finally, fluid accumulation has been linked to increased intra-abdominal pressure and, in severe cases, abdominal compartment syndrome. Elevated intra-abdominal pressure compromises perfusion to intra- and extra-abdominal organs and impairs diaphragmatic excursion, further exacerbating respiratory dysfunction (22).
Taken together, these findings underscore that fluid accumulation is not a benign side effect of surgery but a modifiable risk factor with direct consequences on recovery, morbidity, and resource utilization.
When to De-Resuscitate
Deciding when to shift from fluid administration to active removal relies on clinical judgment rather than a single gold-standard test. In most practices, de-resuscitation is considered once cumulative fluid balance is positive and signs of organ failure are seen—and when perfusion targets have been achieved i.e. falling lactate levels, no clinical signs for tissue hypoperfusion (4). De-resuscitation measures should be started independent of vasoactive medication doses.
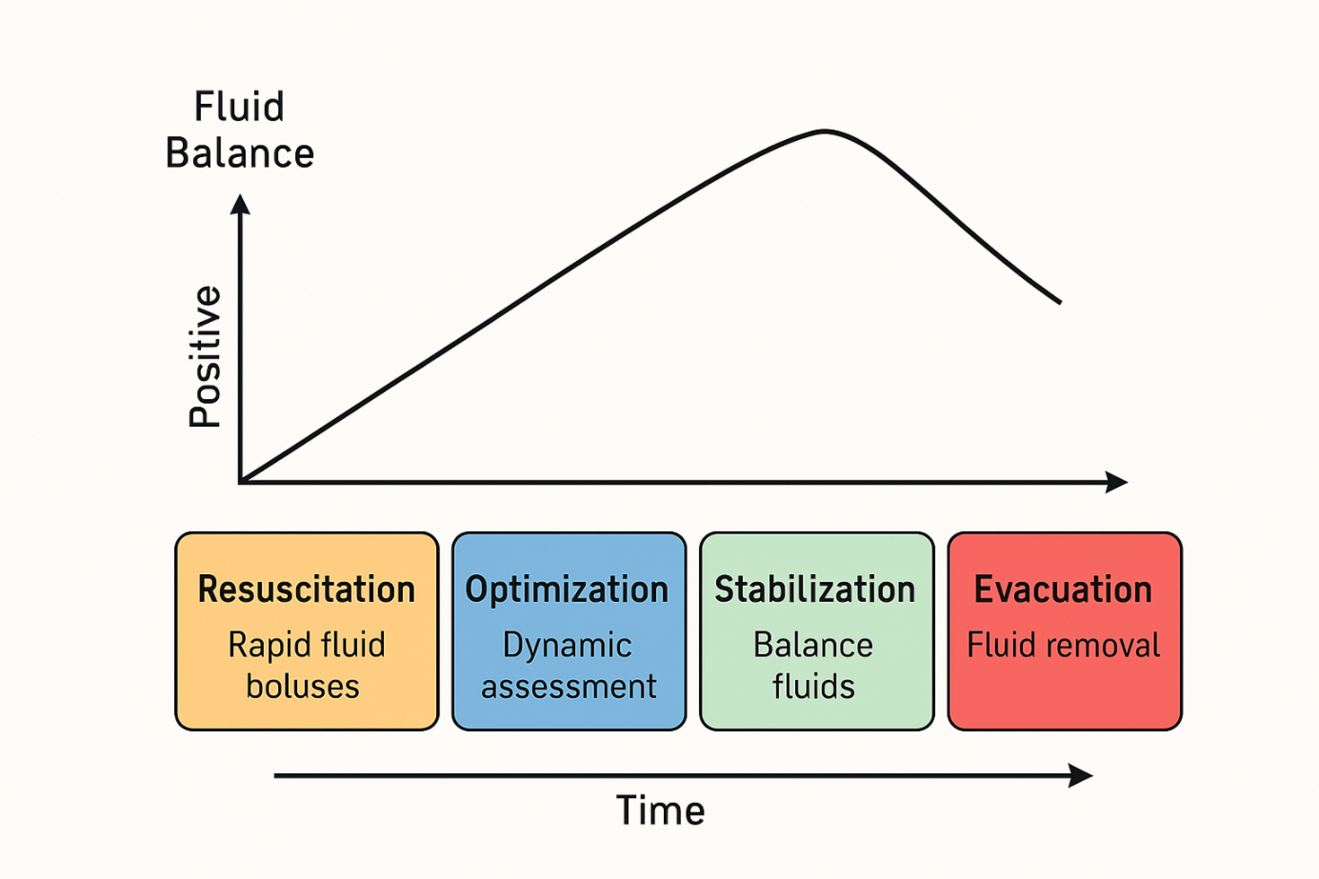
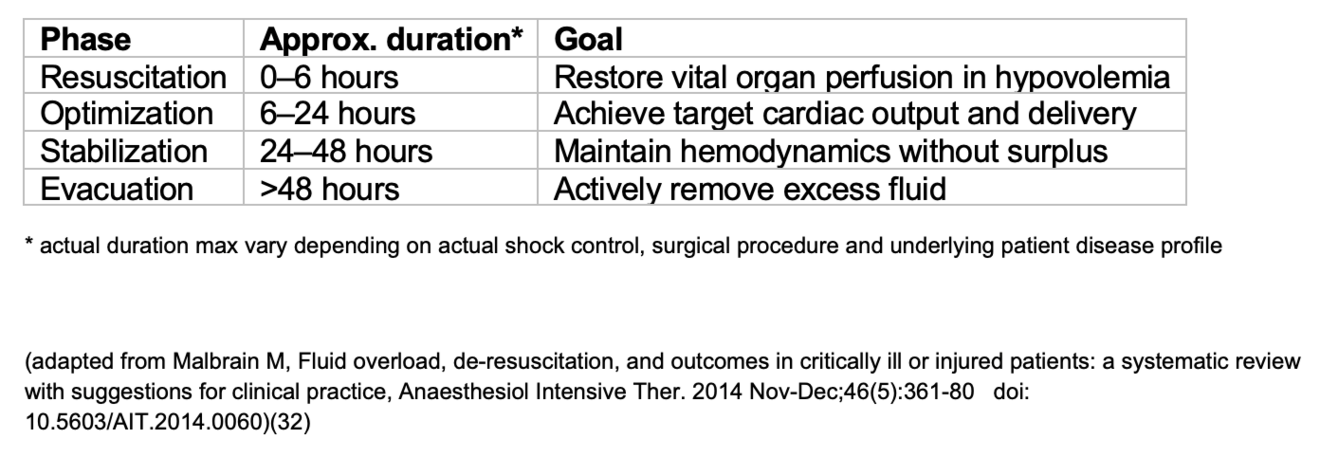
The ROSE model describes four sequential phases of fluid management—Resuscitation, Optimization, Stabilization, and Evacuation—that help clinicians anticipate when to begin fluid removal (Figure 1) (4, 9).
In the Resuscitation phase, rapid fluid boluses restore organ perfusion in life-threatening hypovolemia (4). During Optimization, fluids and vasopressors are adjusted to achieve target cardiac output and oxygen delivery (4). In the Stabilization phase, inputs and outputs are balanced to maintain achieved hemodynamics without inducing deficits or surplus (4). Finally, the Evacuation (De-escalation) phase shifts focus to active removal of excess fluid—through diuretics, ultrafiltration, or mechanical means—once capillary leak subsides and perfusion remains stable (4, 24, 25).
Recognizing when you have moved out of Stabilization into Evacuation is key—this is the trigger to stop routine fluid administration and begin fluid removal. Key clinical clues indicating relevant fluid accumulation include rising edema (pitting swelling of ankles, fingers or eyelids), respiratory changes (crackles at lung bases or increasing oxygen needs), abdominal tension (distended belly or elevated pressures) and sluggish gut recovery (persistent bloating or delayed bowel function) (6, 8, 19, 22). Bedside tools such as lung ultrasound or chest X-ray can confirm pulmonary fluid but are not mandatory; clinical judgment based on these observations and charted balances typically suffices to initiate fluid removal.
How to De-Resuscitate
De-resuscitation combines pharmacological, extracorporeal, and mechanical strategies to mobilize and eliminate excess interstitial fluid, also see table 3.
– Loop Diuretics Continuous furosemide infusion delivers steady diuresis and avoids peaks and troughs of bolus dosing (24, 26). In diuretic-resistant patients, add thiazides (e.g., hydrochlorothiazide) or carbonic anhydrase inhibitors (e.g., acetazolamide) to potentiate natriuresis while closely monitoring electrolytes and hemodynamics (27-29).
– Ultrafiltration/CRRT In oliguria or diuretic intolerance, controlled ultrafiltration via continuous renal replacement therapy (CRRT) or slow-extended daily dialysis (SLEDD) removes fluid at a set rate (100–200 mL/h), preserving hemodynamic stability (20, 25).
– Mechanical Mobilization and Compression Early ambulation activates the skeletal muscle pump, enhancing venous and lymphatic return (18). Graduated compression garments or intermittent pneumatic devices have reduced net positive fluid balance by several hundred milliliters per day in critical illness, potentially benefiting postoperative patients (23, 30).
– Fluid Stewardship Minimize “fluid creep” from IV medications, blood products, and nutrition by applying the four D’s—Drug choice, Dose, Duration, De-escalation—and using protocols (e.g., REDUCE) to standardize de-resuscitation thresholds and monitoring (4, 31).
Conclusion
De-resuscitation after major surgery is a critical component of perioperative care that addresses the detrimental effects of fluid accumulation. Understanding the pathophysiology of capillary leak and lymphatic dysfunction highlights the importance of timely intervention. The ROSE framework guides clinicians through phases of fluid management, while clinical triggers are used to guide fluid removal. Pharmacologic and mechanical modalities, tailored to patient hemodynamics, enable safe and effective de-resuscitation. Future research should refine dynamic monitoring tools and protocols to optimize individualized fluid stewardship and improve surgical outcomes.
- Messmer AS, Zingg C, Müller M, Gerber JL, Schefold JC, Pfortmueller CA. Fluid Overload and Mortality in Adult Critical Care Patients-A Systematic Review and Meta-Analysis of Observational Studies. Critical care medicine. 2020.
- Pfortmueller CA, Dabrowski W, Wise R, van Regenmortel N, Malbrain M. Fluid accumulation syndrome in sepsis and septic shock: pathophysiology, relevance and treatment-a comprehensive review. Ann Intensive Care. 2024;14(1):115.
- Zampieri FG, Bagshaw SM, Semler MW. Fluid Therapy for Critically Ill Adults With Sepsis: A Review. Jama. 2023;329(22):1967-80.
- Malbrain M, Van Regenmortel N, Saugel B, De Tavernier B, Van Gaal PJ, Joannes-Boyau O, et al. Principles of fluid management and stewardship in septic shock: it is time to consider the four D's and the four phases of fluid therapy. Annals of intensive care. 2018;8(1):66.
- Heming N, Moine P, Coscas R, Annane D. Perioperative fluid management for major elective surgery. Br J Surg. 2020;107(2):e56-e62.
- Monnet X, Cipriani F, Camous L, Sentenac P, Dres M, Krastinova E, et al. The passive leg raising test to guide fluid removal in critically ill patients. Annals of intensive care. 2016;6(1):46.
- Curry FE, Michel CC. The Colloid Osmotic Pressure Across the Glycocalyx: Role of Interstitial Fluid Sub-Compartments in Trans-Vascular Fluid Exchange in Skeletal Muscle. Front Cell Dev Biol. 2021;9:729873.
- Dull RO, Hahn RG. Hypovolemia with peripheral edema: What is wrong? Critical Care. 2023;27(1):206.
- Prowle JR, Kirwan CJ, Bellomo R. Fluid management for the prevention and attenuation of acute kidney injury. Nature reviews Nephrology. 2014;10(1):37-47.
- Weinbaum S, Tarbell JM, Damiano ER. The structure and function of the endothelial glycocalyx layer. Annual review of biomedical engineering. 2007;9:121-67.
- Belavic M, Sotosek Tokmadzic V, Fisic E, Brozovic Krijan A, Strikic N, Loncaric Katusin M, et al. The effect of various doses of infusion solutions on the endothelial glycocalyx layer in laparoscopic cholecystectomy patients. Minerva anestesiologica. 2018;84(9):1032‐43.
- Aldrich MB, Sevick-Muraca EM. Cytokines are systemic effectors of lymphatic function in acute inflammation. Cytokine. 2013;64(1):362-9.
- Levick JR, Michel CC. Microvascular fluid exchange and the revised Starling principle. Cardiovasc Res. 2010;87(2):198-210.
- Murphy EL, Kwaan N, Looney MR, Gajic O, Hubmayr RD, Gropper MA, et al. Risk factors and outcomes in transfusion-associated circulatory overload. Am J Med. 2013;126(4):357.e29-38.
- Van Regenmortel N, Verbrugghe W, Roelant E, Van den Wyngaert T, Jorens PG. Maintenance fluid therapy and fluid creep impose more significant fluid, sodium, and chloride burdens than resuscitation fluids in critically ill patients: a retrospective study in a tertiary mixed ICU population. Intensive Care Medicine. 2018;44(4):409-17.
- Wireko BM, Bowling T. Enteral tube feeding. Clin Med (Lond). 2010;10(6):616-9.
- Bankhead R, Boullata J, Brantley S, Corkins M, Guenter P, Krenitsky J, et al. Enteral nutrition practice recommendations. JPEN J Parenter Enteral Nutr. 2009;33(2):122-67.
- Grist G, Whittaker C, Merrigan K, Fenton J, Worrall E, O'Brien J, et al. The correlation of fluid balance changes during cardiopulmonary bypass to mortality in pediatric and congenital heart surgery patients. The journal of extra-corporeal technology. 2011;43(4):215-26.
- Silversides JA, McMullan R, Emerson LM, Bradbury I, Bannard-Smith J, Szakmany T, et al. Feasibility of conservative fluid administration and deresuscitation compared with usual care in critical illness: the Role of Active Deresuscitation After Resuscitation-2 (RADAR-2) randomised clinical trial. Intensive care medicine. 2022;48(2):190-200.
- Kumar VA, Craig M, Depner TA, Yeun JY. Extended daily dialysis: A new approach to renal replacement for acute renal failure in the intensive care unit. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2000;36(2):294-300.
- Messmer AS, Zingg C, Müller M, Gerber JL, Schefold JC, Pfortmueller CA. Fluid Overload and Mortality in Adult Critical Care Patients-A Systematic Review and Meta-Analysis of Observational Studies. Critical care medicine. 2020;48(12):1862-70.
- Rajendram R, Prowle JR. Venous congestion: are we adding insult to kidney injury in sepsis? Critical care (London, England). 2014;18(1):104.
- Baish JW, Padera TP, Munn LL. The effects of gravity and compression on interstitial fluid transport in the lower limb. Sci Rep. 2022;12(1):4890.
- Côté JM, Bouchard J, Murray PT, Beaubien-Souligny W. Diuretic strategies in patients with resistance to loop-diuretics in the intensive care unit: A retrospective study from the MIMIC-III database. Journal of critical care. 2021;65:282-91.
- Rosner MH, Ostermann M, Murugan R, Prowle JR, Ronco C, Kellum JA, et al. Indications and management of mechanical fluid removal in critical illness. British journal of anaesthesia. 2014;113(5):764-71.
- Bouchard J, Mehta RL. Volume management in continuous renal replacement therapy. Seminars in dialysis. 2009;22(2):146-50.
- Mullens W, Dauw J, Martens P, Verbrugge FH, Nijst P, Meekers E, et al. Acetazolamide in Acute Decompensated Heart Failure with Volume Overload. The New England journal of medicine. 2022;387(13):1185-95.
- van IMM, Buter H, Kingma WP, Koopmans M, Navis G, Boerma EC. Hydrochlorothiazide in intensive care unit-acquired hypernatremia: A randomized controlled trial. Journal of critical care. 2017;38:225-30.
- Imiela T, Budaj A. Acetazolamide as Add-on Diuretic Therapy in Exacerbations of Chronic Heart Failure: a Pilot Study. Clin Drug Investig. 2017;37(12):1175-81.
- Dargent A, Large A, Soudry-Faure A, Doise JM, Abdulmalak C, Jonval L, et al. Corporeal Compression at the Onset of Septic shock (COCOONs): a compression method to reduce fluid balance of septic shock patients. Sci Rep. 2019;9(1):11566.
- Messmer A PC. Protocolised Early De-Resuscitation in Septic Shock (REDUCE) clinicaltrials.gov2021 [Available from: https://clinicaltrials.gov/ct2/show/NCT04931485
- Malbrain ML, Marik PE, Witters I, Cordemans C, Kirkpatrick AW, Roberts DJ, et al. Fluid overload, de-resuscitation, and outcomes in critically ill or injured patients: a systematic review with suggestions for clinical practice. Anaesthesiol Intensive Ther. 2014;46(5):361-80.