Initially described as merely theoretical therapy for lymphedema, lymphovenous anastomosis has become a widely approved procedure with a constantly increasing range of applications. The improvements of supermicrosurgical instruments and techniques allow nowadays to work safely with always smaller and more distal vessels, which makes this procedure suitable not only for the treatment of early-stage lymphedema, but also for moderate-to-severe disease, and as a preventive measure in case of lymphatic impairments.
In the early sixties, at the beginning of the microsurgery history, the lymphovenous anastomosis (LVA) was firstly mentioned in 1962 by Jacobson and Suarez in a canine model1 . Then, other authors proposed its application for the treatment of primary and secondary lymphedema in humans2-3-4. The first case series dates back to 1977 when O’Brien et al. shared their experience with microsurgical lymphovenous bypass in human upper limbs5 . However, at that time, the results were often poor and inconsistent, mostly because of high-pressure veins and low-quality lymphatic vessels6-7.
Over the last 60 years huge advances were made from technical and anatomical points of view. These aspects regard a better anatomic and physiologic understanding of lymphatic and venous systems, and with many technological improvements. In particular pre- and intra-operative imaging, operating microscopes and surgical instruments, allowed us to perform safely anastomoses with vessels smaller than 0.8 mm in diameter, thus entering into the supermicrosurgical field. As a consequence, it became possible to work on more distal and more superficial vessels, where lowpressure venules are easily available, thus offering a more favorable pressure gradient.
Surgical technique
LVA represents a surgical procedure intended to physiologically restore the lymphatic drainage. It consists in diverting the lymph flow from a damaged lymphatic pathway towards the venous vascular network. To reach this, one or more lymphatic vessels are anastomosed to low-pressure nearby veins, distally to the damaged area.
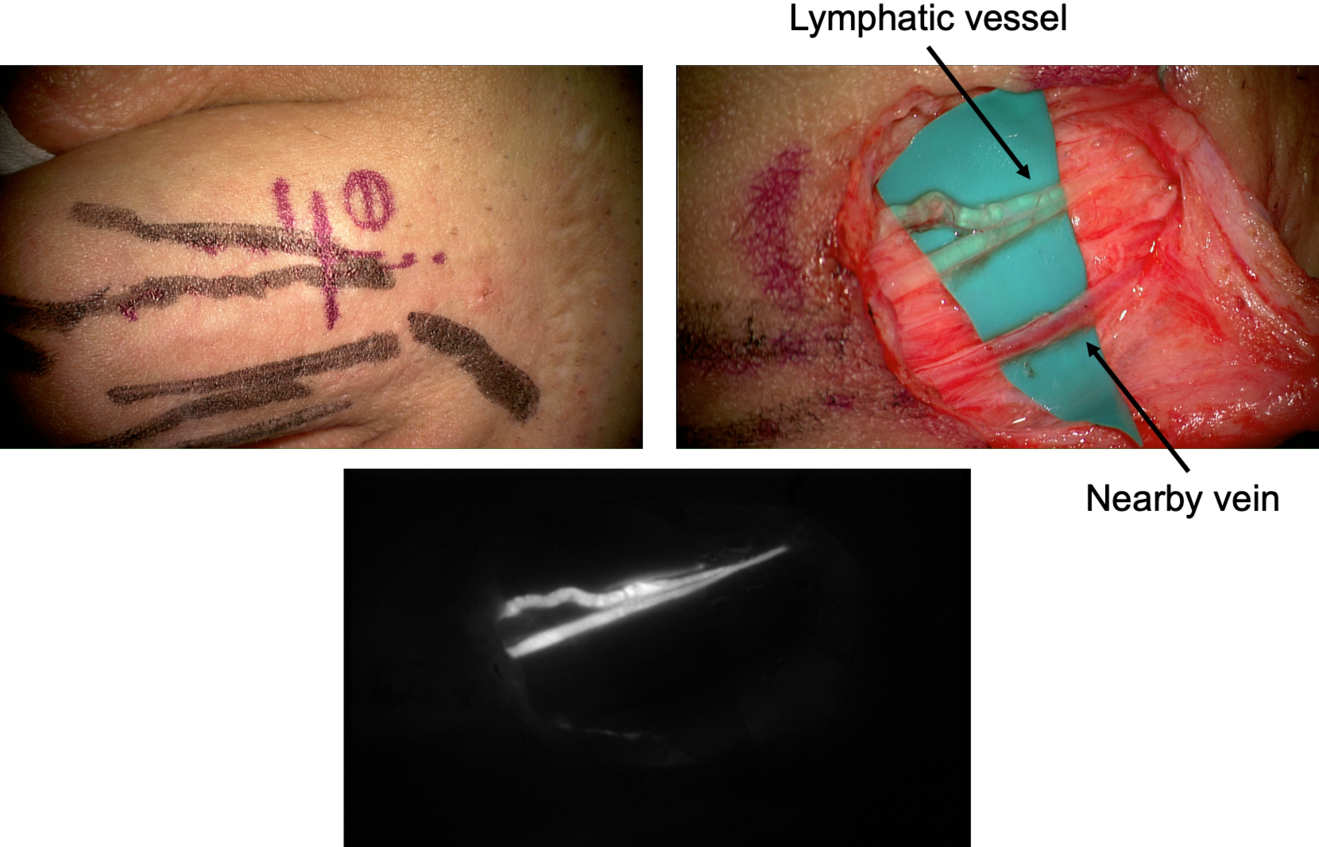
A crucial step for a successful result is the pre-operative vessel mapping. To do so, we always resort to ICG lymphography, with multiple distal superficial dye injections, which then guide us during the skin markings. The anatomy of the superficial lymphatic system is normally closely related to the venous one8 . Therefore, in our experience a nearby vein is usually available, however, infrared veins’ mapping may also be useful the pre-operative setting. In case of severe lymphedema, ICG lymphography may also allow identification of lymphostatic choke points, with the so-called dermal backflow patterns. In this case the anastomosis should be performed just proximal to that point (Figure 1).
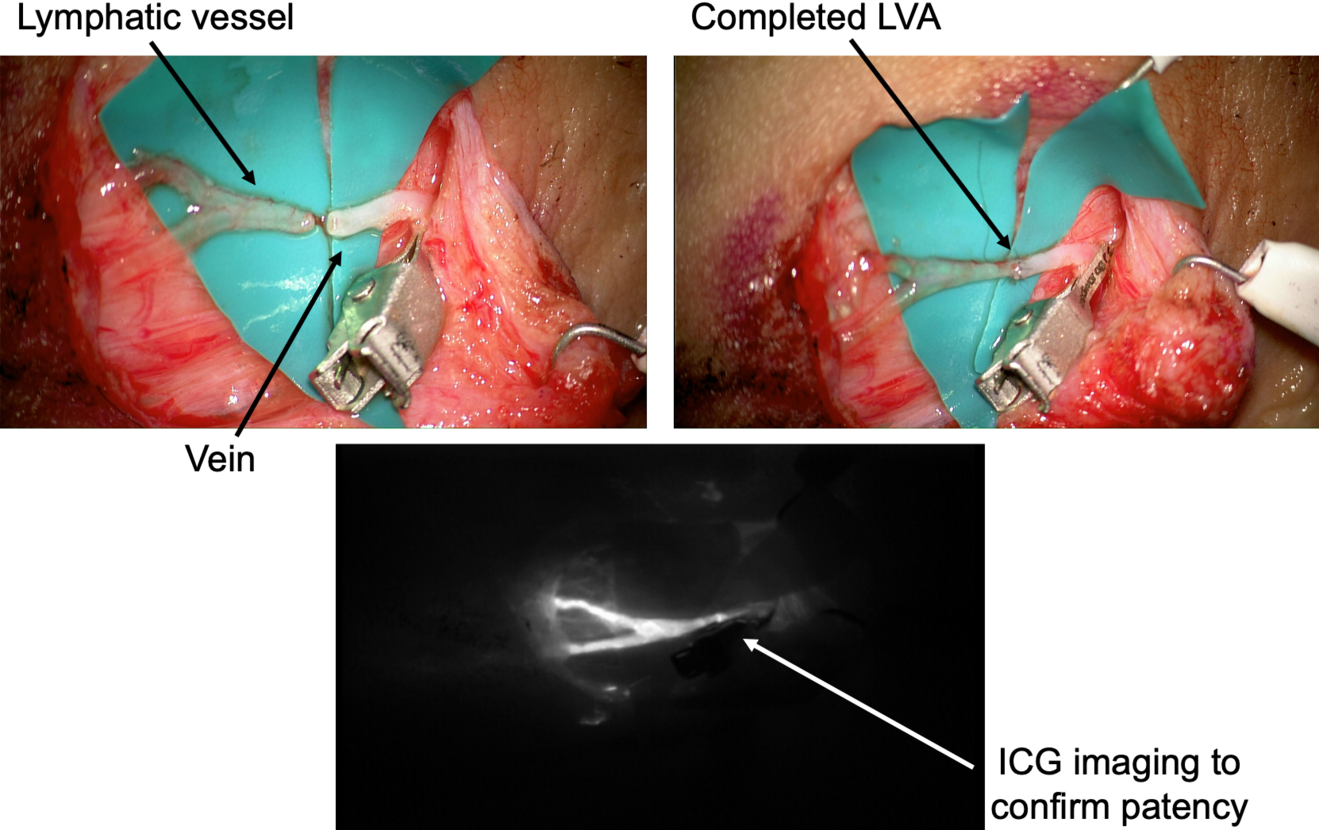
A perpendicular 2cm incision is performed at selected site, followed by a caudo-cephalic dissection under 20-25x microscope magnification. When the vessels are properly isolated, their function is evaluated, and they are staged as healthy, ectatic, contracted, or sclerotic9 . This step has a considerable impact since it may result in a different type of anastomosis. A wide variety of techniques are known so far, such as single or multiple end-toend anastomosis, end-to-side or side-to-side anastomosis, and invaginating «octopus» anastomosis (consisting in multiple small caliber lymphatics invaginated into a single large-caliber vein), each of them with specific indications and limits.
End-to-end fashion is the most common and easier to perform type, and it represents the gold-standard in almost every case if a proper pre-operative vessels’ selection is made. However, there are some conditions where other solutions may provide better results. Either side-to-side or end-to-side anastomosis are is usually chosen when the pressure gradient is unfavorable, while the «octopus» technique is appropriate when only fibrotic lymphatics are available since it allows linkage to a large number of vessels to a single larger vein.
After the anastomosis, its patency must be confirmed. In our experience, we routinely check the washout into the venous lumen by means of intra-operative ICG lymphography under the microscope (Figure 2). This process might be delayed, but it is crucial to be confirmed in order to reach a successful result. If a backflow towards the lymphatic vessel is observed, the risk of thrombosis is significantly increased and the efficacy of the anastomosis is extremely limited.
One of the main criticisms addressed to LVA so far is the long-term patency of the anastomoses. In this perspective, Joost et al.10 have done a particularly interesting work analyzing not only the efficacy of this procedure in terms of improvement of signs and symptoms, but also measuring the actual number of patients presenting a conserved patency after 12 months, and its positive correlation with clinical improvements. They showed that more than 70% of the patients still had at least one patent anastomosis, and, even more interesting, that this percentage increased with increasing number of anastomoses, up to 100% for those who received at least 3 anastomoses.
In literature there is still a significant variation regarding the ideal number of anastomoses, and the necessity of supplementary interventions11. Therefore, this element may help in giving a clear indication about the adequate number of anastomoses to be performed in each patient. Previous studies also tried to tackle this point, but no consensus was obtained, with some authors sustaining the importance of a high number of anastomoses and others stating that this aspect does not significantly influence the efficacy of the treatment12. In our experience, we believe that a few high-quality anastomoses are sufficient to provide a relevant improvement in terms of lymphatic drainage and symptoms relief. For this reason, we usually perform 3 to 5 LVAs per case.
Clinical applications
LVA was traditionally considered only for early stage, fluid-predominant lymphedema (I-II according to the ISL staging of lymphedema). However, with the recent improvements and technical refinements, this technique also showed encouraging results in more advanced phases of the disease. This aspect expanded the spectrum of applications, which spans from secondary lymphedema, to primary lymphedema, and also to prophylactic surgery.
The most common employment of LVA remains the treatment of secondary lymphedema, in both upper and lower limbs. In this context, according to the severity of the disease, it might be used either alone or combined with other procedures, such as suctionassisted lipectomy and ablative surgeries. In this case the debulking surgery is intended to remove the excessive tissues, while LVA is responsible for the restoration of an adequate lymphatic drainage. Another frequent indication for LVAs is the treatment of iatrogenic lymphocele. In this case, diverting the lymphatic flow allows us to decrease the amount of fluid reaching the affected area, resulting in a significant improvement of recurrency rate, even in severe cases13.
Among the newer applications of LVA there is definitely the treatment of primary lymphedema. One of the main reasons why LVA was considered to be contraindicated in this setting was related to the theoretical concerns that the abnormal structure and functions of the lymphatic vessels may decrease the efficacy of LVA. However, it has been demonstrated that these patients often present a systemic lymphatic dysfunction, which precludes the possibility of performing other, more invasive, procedures, leaving LVA as the safer alternative14. Hara et al. in 2015 presented an interesting work including 62 patients affected by lower limb primary lymphedema, which showed a significant decrease in volume after LVA in patients with an age of onset age higher than 11 years old. Moreover, the presence of lymphedema for a longer period did not negatively impact the lymphaticovenous anastomosis efficacy15.
Another fascinating field where LVA is gaining popularity is the prevention of lymphedema, in particular after lymphatic dissection or massive oncologic surgeries. Initially described by Boccardo et al. in 2009 with the acronym LYMPHA (Lymphatic Microsurgical Preventive Healing Approach), this prophylactic procedure consisted in performing multiple LVAs at the same time as that of lymph node dissection16. However, over the years many other researches have been made in this field and new interesting prophylactic surgeries have been proposed.
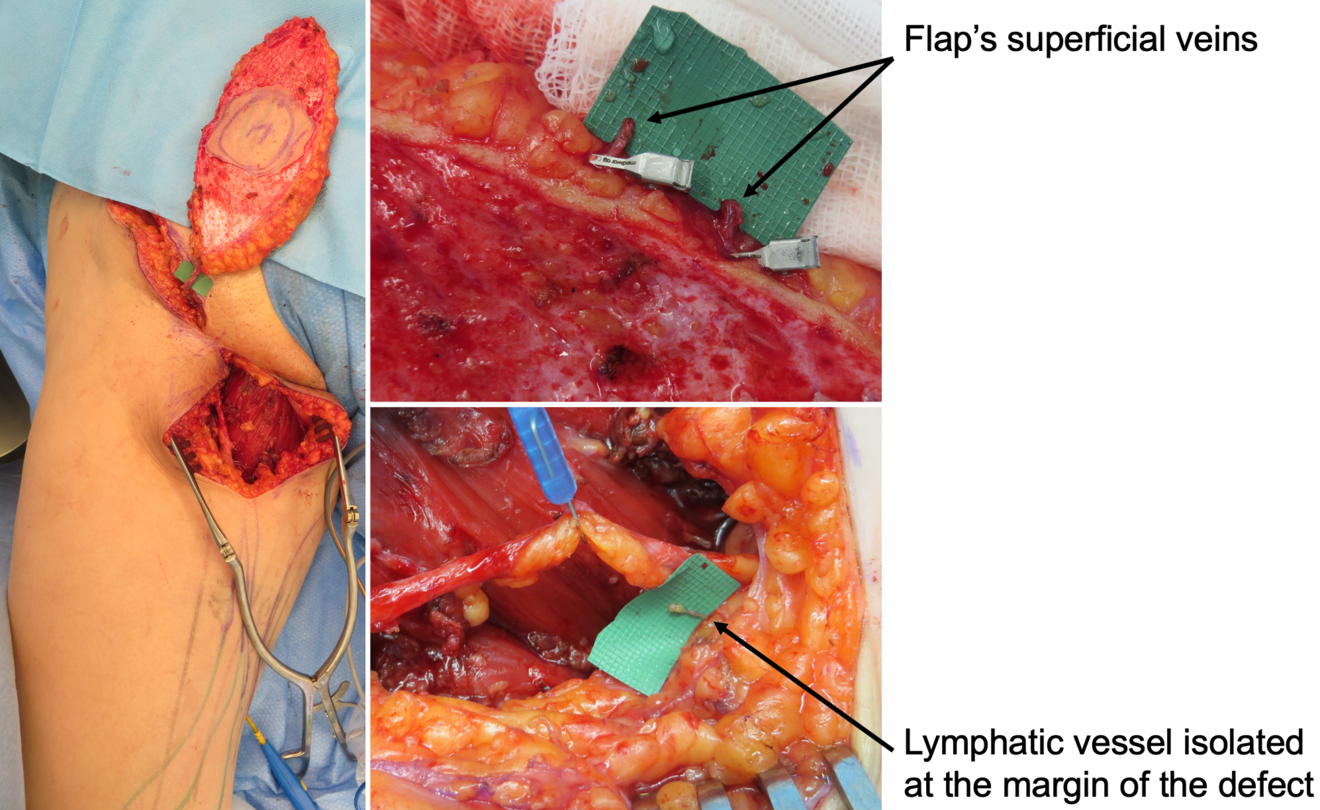
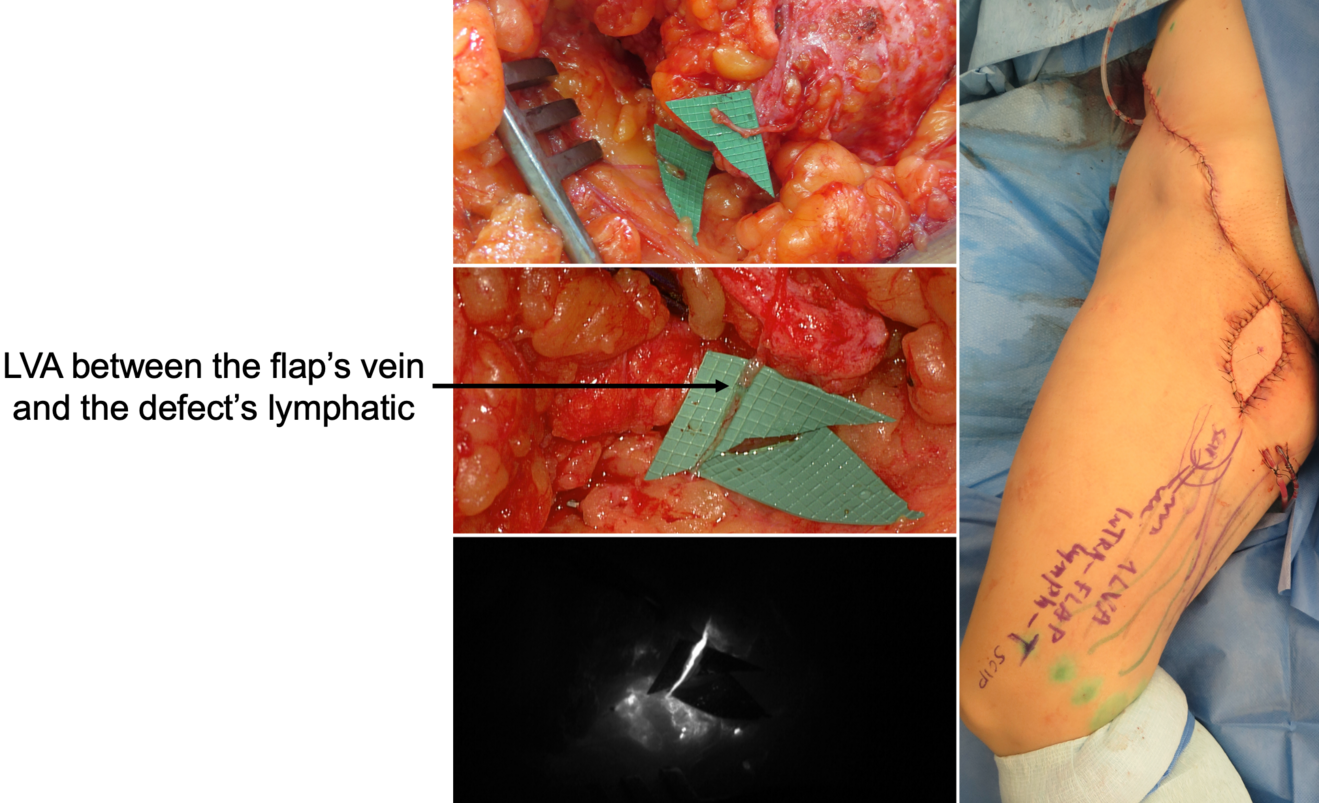
In our practice, we built a rather significative experience with lymphedema prevention after large sarcoma resections. Lymphatic impairment is not only due to lymphnode dissection but it may be also caused by the removal of lymphatic-rich tissues, such as in the upper medial thigh region. For this reason, we started to combine the reconstruction of the defect with LVA, performed distally to the defect itself, obtaining encouraging results in terms of lymphatic complications reduction (Figure 3)17. Then, another recently-developed technique in this setting is the lymphatic flowthrough (LyFT) concept. It relies on superficial veins coming from the donor flap as recipient vessels for additional LVAs, which are performed at the recipient site (Figure 4-5). Since it employs only healthy veins coming from an unaffected area, this solution is particularly useful in case of adjuvant radiotherapy or severely depleted surgical sites18.
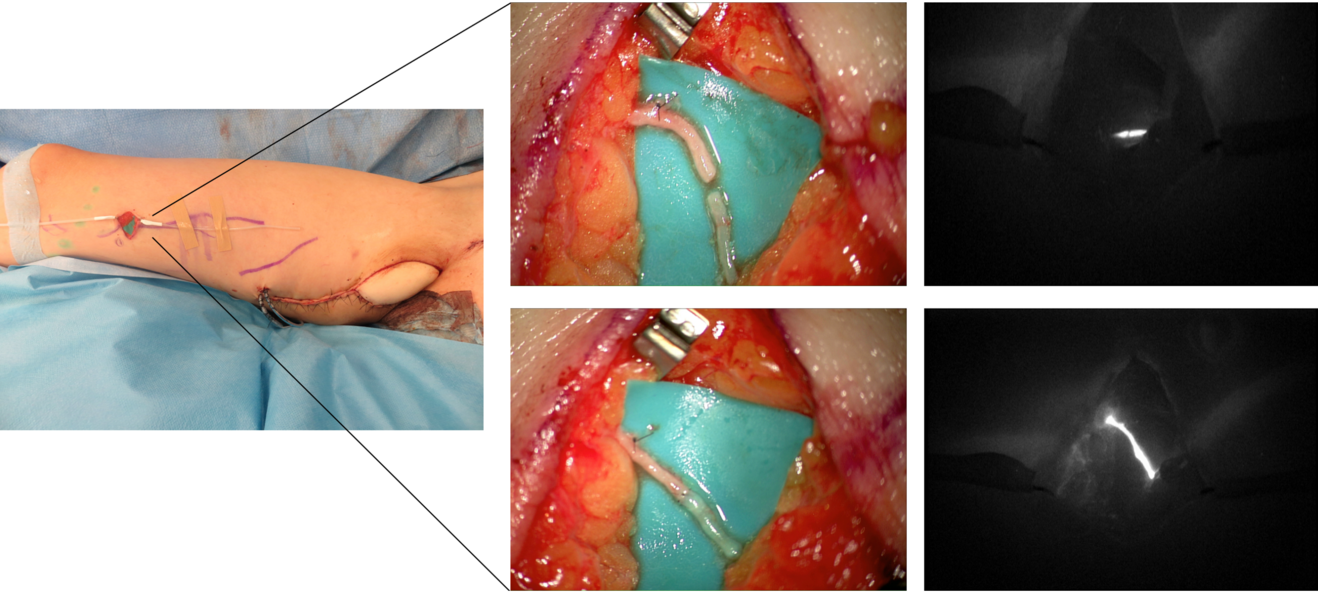
Finally, considering the safety that this technique has reached nowadays, it is worth mentioning the fact that more and more unconventional applications of LVA are being described in the most disparate settings. For example, we recently published an interesting case report where multiple LVAs have been used to successfully treat a rare case of isolated penile lymphedema unresponsive to conservative treatments (Figure 1-2)19.
Future of LVA
In recent years became clear that LVA is clearly effective in providing both subjective and objective improvement of lymphedemaassociated symptoms, including the decrease of tissue excess. Moreover, its minimally invasive nature, compared to other lymphatic surgeries such as vascularized lymph node transfer (VLNT) or vascularized lymphatic vessels transfer (VLVT), often outweighs its drawbacks.
As demonstrated by Yang et al.20 and by other high-volume centers21 has demonstrated that in hands of experienced microsurgeons, LVA can be safely employed also in moderate-to severe diseases, further widening its range of application and creating an overlap of indications with VLN/VLVT.
Then, the increasing attention to lymphatic complications made the LVA particularly suitable as a prophylactic procedure, and it will be always more often performed simultaneously with large and complex reconstructive procedures. For this reason, we believe that in the future this micro- and even supermicrosurgical skills will became always more important in the technical armamentarium of plastic surgeons.
- Jacobson JH, Suarez EL. Microvascular surgery. Dis Chest 1962;41:220-4
- Laine J, Howard J. Experimental lymphatico-venous anastomosis. Surg Forum 1963;14:111-2
- Nielubowicz J, Olszewski W. Surgical lymphaticovenous shunts in patients with secondary lymphoedema. Br J Surg 1968;55:440-2
- Yamada Y. The studies on lymphatic venous anastomosis in lvmphedema lymphedema. Nagoya J Med Sci 1969;32:1-21
- O‘Brien BM, Sykes P, Threlfall GN, Browning FS. Microlymphaticovenous anastomoses for obstructive lymphedema. Plast Reconstr Surg 1977;60:197-211
- Mihara M, Hara H, Hayashi Y, et al. Pathological steps of cancer-related lymphedema: histological changes in the collecting lymphatic vessels after lymphadenectomy. PLoS One 2012;7:e41126
- Mihara M, Hara H, Kawakami Y, et al. Site specific evaluation of lymphatic vessel sclerosis in lower limb lymphedema patients. Lymphat Res Biol 2018;16:360-7
- Pan WR, Wang DG, Levy SM, Chen Y. Superficial lymphatic drainage of the lower extremity: anatomical study and clinical implications. Plast Reconstr Surg 2013;132:696-707
- Hara H, Mihara M, Seki Y, Todokoro T, Iida T, Koshima I. Comparison of indocyanine green lymphographic findings with the conditions of collecting lymphatic vessels of limbs in patients with lymphedema. Plast Reconstr Surg 2013;132:1612-8
- Wolfs JAGN, de Joode LGEH, van der Hulst RRWJ, Qiu SS. Correlation between patency and clinical improvement after lymphaticovenous anastomosis (LVA) in breast cancer-related lymphedema: 12-month follow-up. Breast Cancer Res Treat. 2020;179(1):131-138
- Scaglioni MF, Fontein DBY, Arvanitakis M, Giovanoli P. Systematic review of lymphovenous anastomosis (LVA) for the treatment of lymphedema. Microsurgery. 2017;37(8):947-953
- Chang DW, Suami H, Skoracki R. A prospective analysis of 100 consecutive lymphovenous bypass cases for treatment of extremity lymphedema. Plast Reconstr Surg. 2013;132(5):1305-1314
- Scaglioni MF, Meroni M, Fritsche E. Lymphovenous anastomosis (LVA) for treatment of iatrogenic lymphocele in the thigh. Microsurgery. 2021;41(1):19-25
- Chen WF, McNurlen M, Bowen M. Surgical treatments of lymphedema. In: Jones GE, editor. Bostwick’s plastic and reconstructive breast surgery. Thieme Medical Publishers; 2020. p. 1478-99
- Hara H, Mihara M, Ohtsu H, Narushima M, Iida T, Koshima I. Indication of Lymphaticovenous Anastomosis for Lower Limb Primary Lymphedema. Plast Reconstr Surg. 2015;136(4):883-893
- Boccardo F, Casabona F, De Cian F, et al. Lymphedema microsurgical preventive healing approach: a new technique for primary prevention of arm lymphedema after mastectomy. Ann Surg Oncol. 2009;16(3):703-708
- Scaglioni MF, Meroni M, Fritsche E, Fuchs B. Combined pedicled superficial circumflex iliac artery perforator (SCIP) flap with lymphatic tissue preservation and lymphovenous anastomosis (LVA) for defect reconstruction and lymphedema-lymphocele prevention in thigh sarcoma surgery: Preliminary results. J Surg Oncol. 2021;123(1):96-103
- Scaglioni MF, Meroni M, Fritsche E. Soft Tissue Defect Reconstruction and Lymphatic Complications Prevention: The Lymphatic Flow-Through (LyFT) Concept. Medicina (Kaunas). 2022;58(4):509
- Scaglioni MF, Meroni M, Fritsche E. Lymphovenous anastomosis (LVA) for treatment of isolated penile lymphedema: A case report. Microsurgery. 2020;40(6):692-695
- Yang JC, Wu SC, Lin WC, Chiang MH, Chiang PL, Hsieh CH. Supermicrosurgical lymphaticovenous anastomosis as alternative treatment option for moderate-to-severe lower limb lymphedema. J Am Coll Surg 2020;230:216-27
- Pandey SK, Fahradyan V, Orfahli LM, Chen WF. Supermicrosurgical lymphaticovenular anastomosis vs. vascularized lymph vessel transplant - technical optimization and when to perform which. Plastic and Aesthetic Research. 2021