Patients with minor PNI such as digital nerve injuries commonly recover well. In contrast, more extensive PNI, particularly injuries of the brachial or lumbosacral plexus, are a substantial challenge for both patients and peripheral nerve surgeons. Not only are these patients often affected by a devastating loss of motor and sensory function, they are also unfit to work for long periods, which may significantly affect their psychological and socio-economic well-being1.
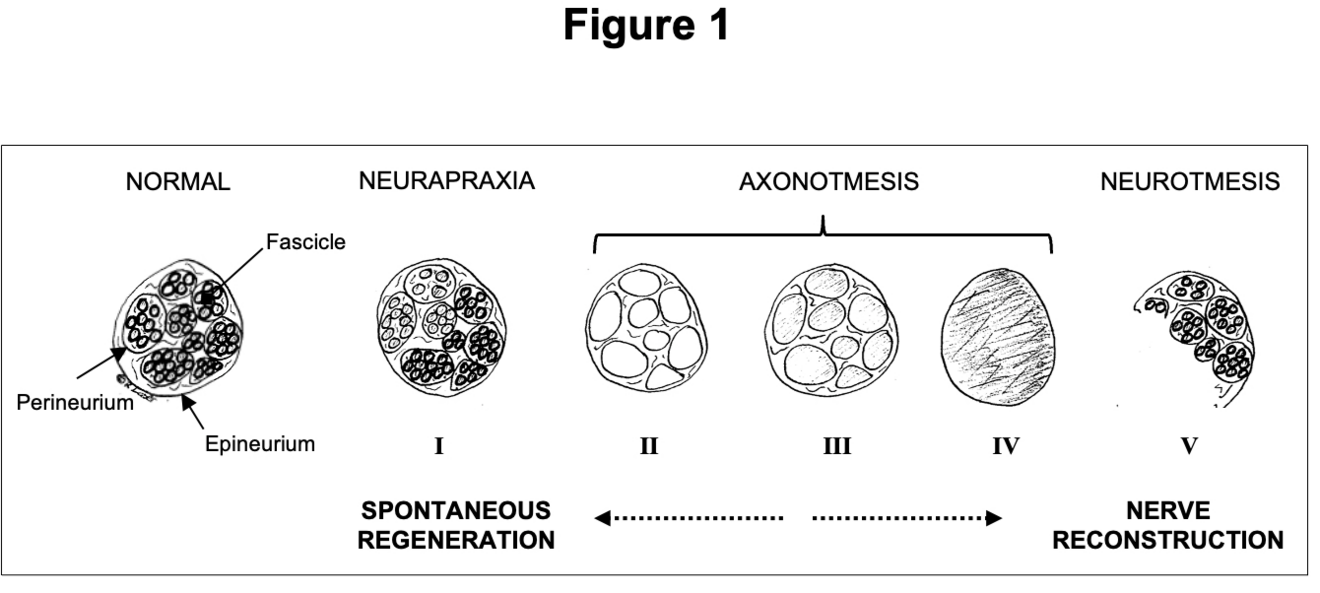
Peripheral nerves are composed of individual fascicles with nerve fibres. These fibres contain axons and Schwan cells, surrounded by a loose extracellular matrix, called endoneurium2. The perineurium surrounds individual fascicles while the more solid epineurium represents the external sheath of a nerve (Fig. 1). The epineurium carries the axial blood and lymphatic vessels nourishing peripheral nerves3. It is also the layer that is microsurgically sutured during PNI repair. Depending on the extent of tissue damage, PNI can be classified as neurapraxia (ie, conduction block), axonotmesis (ie, axonal injury) or neurotmesis (ie, complete nerve transection). Sunderland introduced subgroups of axonal injuries, depending on the involvement of the endoneural or perineural tissue4. Generally, low-degree nerve injuries can undergo spontaneous regeneration whereas higher-degree injuries should be explored and repaired3 (Fig. 1).
Traditional surgical approaches to injuries of the peripheral nerves include primary nerve coaptation or nerve reconstruction using autologous nerves, harvested from the upper or lower extremity. However, in the last decades, peripheral nerve surgeons have explored and introduced novel surgical strategies to enhance the functional outcome of PNI, particularly of the upper extremity. These include but are not limited to allograft nerve repair5 and nerve transfers6. Furthermore, there are several novel surgical interventions to tackle chronic neuropathic pain, which is among the most debilitating complications of a PNI. Among these, targeted muscle reinnervation (TMR) and relocation nerve grafting are particularly interesting for chronic pain patients7,8.
These surgical techniques offer promising options for PNI patients and the modern peripheral nerve surgeon should be familiar with their implementation in the treatment of PNI and neuropathic pain. Since the exposure to major PNI is limited in Switzerland due to a low total case number, surgeons dealing with these complex problems benefit from training in international centers with high-intensity PNI services. In addition, the exchange with the international community is key to develop up-to-date algorithms for PNI patients and to guarantee an adequate diagnostic assessment as well as peri- and postoperative treatment for these demanding injuries.
Peripheral nerve gap reconstruction
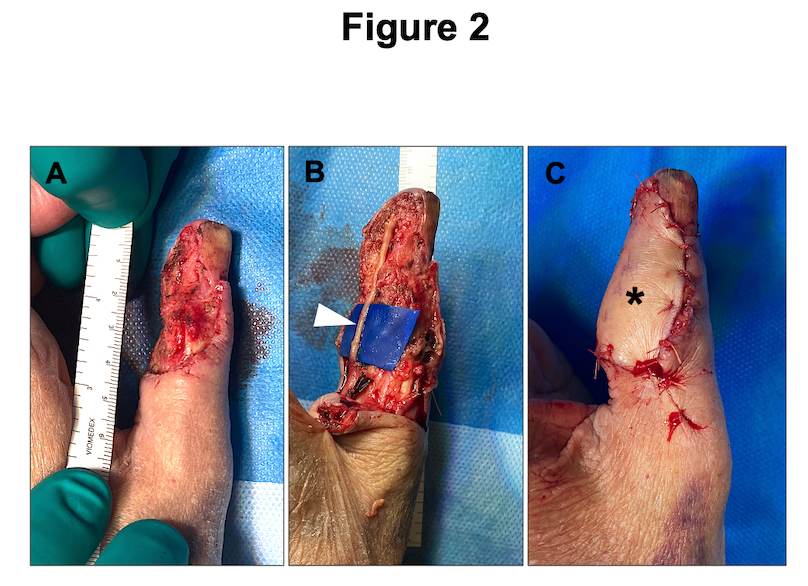
If an injured nerve cannot be repaired directly, reconstruction of the missing segment is required. For this purpose, autologous nerve grafts can be obtained from various donor sites, including the forearm (ie, medial or lateral antebrachial cutaneous nerve, Fig. 2) or the lower leg (ie, sural nerve). This technique has been considered the gold standard in PNI reconstruction for decades9. It is, however, not an ideal solution due to the risk of donor site complications, longer surgery times, and a possible size mismatch10. Peripheral nerve allografts (PNA) are an established alternative to autologous nerve reconstruction, avoiding the before-mentioned downsides11. Currently, there is one PNA available for human nerve defect reconstruction up to 70 mm length (Avance Nerve Graft by Axogen Corporation: https://axogeninc.eu/avance-nerve-graft/). From a biological perspective, PNA are processed with removal of all cell products and preservation of the 3D microarchitecture to support axonal ingrowth and sensory or motor reinnervation12. The Avance Nerve Graft has been successfully used for nerve gap reconstruction with high rates of “meaningful recovery”, corresponding to a satisfactory regeneration of sensory or motor function13. There are, however, important limitations in its suitability for more substantial PNI, affecting motor peripheral nerves or the brachial plexus. Moreover, after PNA implementation in routine nerve reconstruction, reports about suboptimal results have recently been published, raising concerns about the use of PNA for all kind of nerve defects10. Interestingly, there is an ongoing scientific debate about their use and two recent systematic reviews come to different conclusions about the quality of the existing literature as well as the suitability of PNA for nerve reconstruction10,13. In our practice, PNA reconstruction is the first-line treatment for sensory nerve defects up to 50 mm. However, based on the current evidence, we recommend that peripheral nerve surgeons carefully evaluate PNA reconstruction of longer nerve gaps and major motor nerves until a randomised clinical trial confirming PNA non-inferiority versus autologous nerve reconstruction is available.
Peripheral nerve transfers
Proximal PNI, in particular those affecting the brachial plexus, come along with long reinnervation distances and hardly predictable clinical outcomes using traditional nerve grafting techniques. After denervation, the muscular end organ should be reinnervated within 12-18 months before irreversible atrophy and degeneration occurs14. The concept of nerve transfers targets at reinnervating a peripheral muscle by a healthy and expendable donor nerve. First descriptions of peripheral nerve transfers for PNI go back to the early 20th century but in the last decades, multiple novel transfers were introduced to enhance upper and lower extremity nerve reconstruction. They are of high value in cases where proximal reconstruction is not possible (ie, root avulsion) or not reasonable (ie, long-distance nerve defects). Of note, peripheral nerve transfers are also increasingly implemented in the treatment of spinal cord injuries15 and to salvage neurological deficits after soft tissue tumor resection16.
From a biological point of view, nerve transfers offer many benefits14: First, they bring uninjured axons close to a denervated target, converting a proximal PNI to a more distal one. Second, they reduce the risk of motor endplate degradation due to faster reinnervation compared with traditional nerve repair techniques. Third, there is only one nerve coaptation site, limiting the potential for axonal loss due to scarring or fascicular misalignment. Finally, nerve transfers allow operating in an unscarred bed, ensuring more precise anatomic identification, limiting surgery times as well as reducing the risk for iatrogenic injury14.
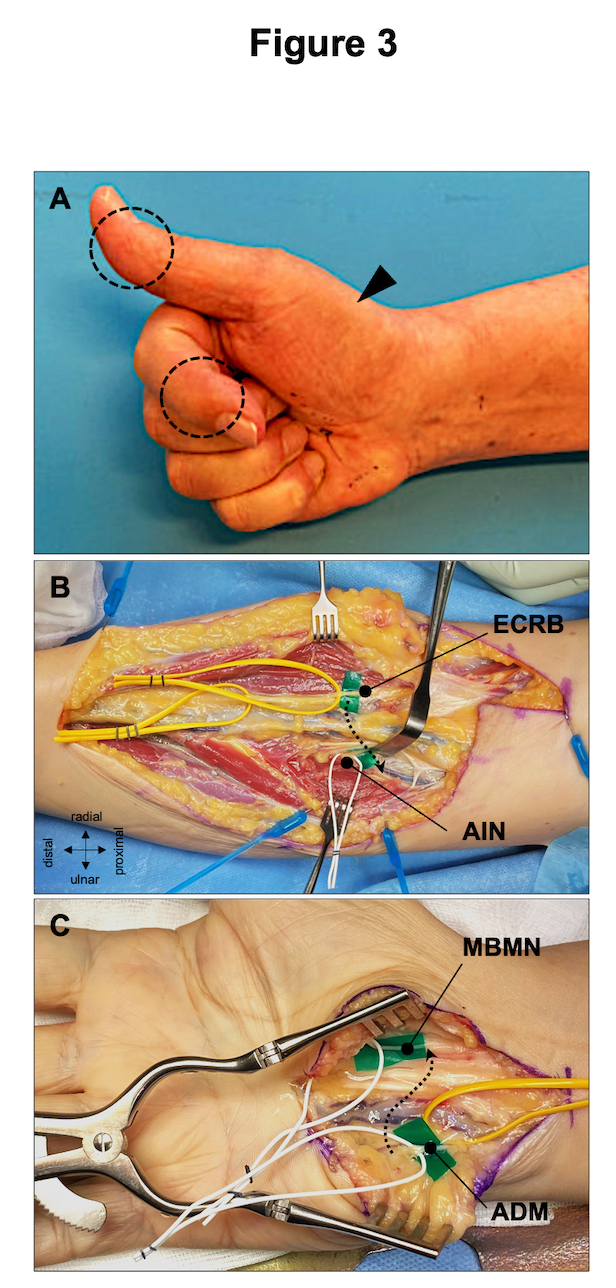
In brachial plexus surgery, the most commonly performed nerve transfers target at the reactivation of elbow flexion, shoulder abduction and external rotation17. Modern reconstructive treatment strategies should always consider the combination of nerve grafting and powerful nerve transfers to provide the denervated limb with as many healthy axons as possible. There are also sophisticated nerve transfers for the treatment of isolated proximal major nerve injuries, such as high median or ulnar nerve palsy (Fig. 3). If these injuries are diagnosed with significant delay, peripheral nerve transfers may be the only option the preserve muscle vitality. Due to the complexity of the preoperative decision-making, we believe that injuries of major peripheral nerves at or proximal to elbow/knee level should be referred to specialized centers offering both traditional nerve repair and nerve transfer techniques.
Surgical treatment of chronic neuropathic pain
The best method to prevent neuropathic pain is a flawless PNI repair or nerve reconstruction. However, after higher-degree PNI repair/reconstruction, healing inevitably comes along with intraneural scarring. Therefore, at the site of a nerve coaptation, a painful neuroma can develop despite a sound technical nerve repair. Among the many long-term sequelae that can affect patients after PNI, chronic neuropathic pain is one of the most challenging problems. Pain can lead to complete functional loss of an extremity with major consequences in a patient´s private and professional life.
Surgeons have tried to tackle chronic neuropathic pain with a multitude of interventions, including neurolysis, neuroma excision/reconstruction, neurectomy, burying affected nerves in muscle or bone, nerve caps, and others18. However, none of these techniques is consistently associated with permanent pain control. In recent years, novel surgical strategies such as targeted muscle reinnervation (TMR) and relocation nerve grafts have been proposed to address painful neuropathic pain. Both techniques provide the regrowing axons a place to go and something to do far from the painful neuroma site19. TMR consists of the coaptation of severed peripheral nerves to a newly divided nearby motor nerve19. In contrast, a relocation nerve graft is a long PNA rerouting nerve fibres from painful stumps out of the zone of concern8. In our experience, both methods are promising to address debilitating chronic neuropathic pain and should be incorporated in the peripheral nerve surgeon´s armamentarium.
Conclusion
The surgical treatment of PNI and chronic neuropathic pain is a challenging and quickly evolving field. In order to develop up-to-date treatment algorithms, peripheral nerve surgeons should master multiple technical and tactical skills. These include the thoughtfully evaluated use of PNA, peripheral nerve transfers and the implementation of the most recent strategies to treat painful neuromas.
- Wu KY, Spinner RJ, Shin AY. Traumatic brachial plexus injury: diagnosis and treatment. Curr Opin Neurol. 2022;35:708-17.
- Maggi SP, Lowe JB 3rd, Mackinnon SE. Pathophysiology of nerve injury. Clin Plast Surg. 2003;30:109-26.
- Frueh FS, Gousopoulos E, Power DM, Ampofo E, Giovanoli P, Calcagni M, et al. A potential role of lymphangiogenesis for peripheral nerve injury and regeneration. Med Hypotheses. 2020;135:109470.
- Sunderland S. A classification of peripheral nerve injuries producing loss of function. Brain. 1951;74:491-516.
- Safa B, Jain S, Desai MJ, Greenberg JA, Niacaris TR, Nydick JA, et al. Peripheral nerve repair throughout the body with processed nerve allografts: Results from a large multicenter study. Microsurgery. 2020;40:527-37
- Moucharafieh RC, Badra MI, Boulos KA, Mansour JI, Daher JC, Wardani HM, et al. Nerve transfers in the upper extremity: A review. Injury. 2020;51:2804-10.
- Dumanian GA, Potter BK, Mioton LM, Ko JH, Cheesborough JE, Souza JM, et al. Targeted muscle reinnervation treats neuroma and phantom pain in major limb amputees: A randomized clinical trial. Ann Surg. 2019;270:238-46.
- Freniere BB, Wenzinger E, Lans J, Eberlin KR. Relocation nerve grafting: A technique for management of symptomatic digital neuromas. J Hand Microsurg. 2019;11:S50-S52.
- Millesi H. Microsurgery of peripheral nerves. Hand. 1973;5:157-60.
- Frostadottir D, Chemnitz A, Johansson Ot LJ, Holst J, Dahlin LB. Evaluation of processed nerve allograft in peripheral nerve surgery: A systematic review and critical appraisal. Plast Reconstr Surg Glob Open. 2023;11:e5088.
- Brooks DN, Weber RV, Chao JD, Rinker BD, Zoldos J, Robichaux MR, et al. Processed nerve allografts for peripheral nerve reconstruction: A multicenter study of utilization and outcomes in sensory, mixed, and motor nerve reconstructions. Microsurgery. 2012;32:1-14.
- Sondell M, Lundborg G, Kanje M. Processed nerve allografts for peripheral nerve reconstruction: A multicenter study of utilization and outcomes in sensory, mixed, and motor nerve reconstructions. Brain Res. 1998;795:44-54.
- Lans J, Eberlin KR, Evans PJ, Mercer D, Greenberg JA, Styron JF. Processed nerve allografts for peripheral nerve reconstruction: A multicenter study of utilization and outcomes in sensory, mixed, and motor nerve reconstructions. Plast Reconstr Surg. 2023;151:814e-827e.
- Rinker B. Nerve transfers in the upper extremity: A practical user's guide. Ann Plast Surg. 2015;74 Suppl 4:S222-8.
- Bazarek S, Brown JM. The evolution of nerve transfers for spinal cord injury. Exp Neurol. 2020;333:113426.
- Jawad AM, Duraku LS, Susini F, Chaudhry T, George S, Jester A, et al. Resect, rewire, and restore: Nerve transfer salvage of neurological deficits associated with soft tissue tumors in a retrospective cohort series at a tertiary reconstructive center. J Plast Reconstr Aesthet Surg. 2023:S1748-6815(23)00203-6.
- Maldonado AA, Bishop AT, Spinner RJ, Shin AY. Five Operations That Give the Best Results after Brachial Plexus Injury. Plast Reconstr Surg. 2017;140:545-56.
- Scott BB, Winograd JM, Redmond RW. Surgical approaches for prevention of neuroma at time of peripheral nerve injury. Front Surg. 2022;9:819608.
- Chappell AG, Jordan SW, Dumanian GA. Targeted muscle reinnervation for treatment of neuropathic pain. Clin Plast Surg. 2020;47:285-93.